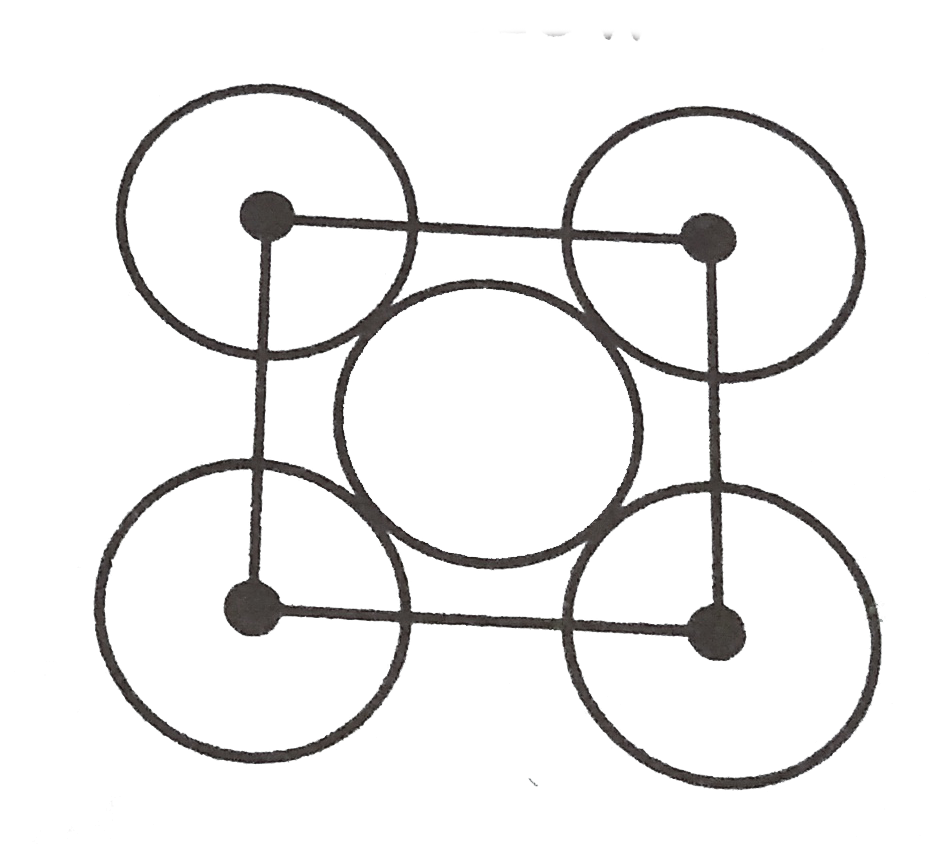
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Objective Questions (Level-B)|26 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Asertion-Reason type Questions|7 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise Practice Problems|20 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise Self Assessment|21 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (SOLID STATE)-OBJECTIVE QUESTIONS (LEVEL -A)
- If a stands for the edge length of the cubic system : simple cubic, b...
Text Solution
|
- Which of the following statement are correct for the ionic solids in w...
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- Lithium forms body centred cubic structure .The length of the side of ...
Text Solution
|
- Which of the following exists as covalent crystals in the solid state?
Text Solution
|
- Experimentally it was found that a metal oxide in formula M(0.98)O. Me...
Text Solution
|
- Why is graphite used as a dry lubricant in machinery ?
Text Solution
|
- Sodium metal crystllizes in a body centred cubic lattice with a unit c...
Text Solution
|
- Which of the following compounds is metallic and ferromagnetic ?
Text Solution
|
- A metal crystallises in a face centred cubic structure. If the edge le...
Text Solution
|
- Cations are present in the interstitial sites in …………… .
Text Solution
|
- If a metal crystallises in a face-centred cubic structure with metalli...
Text Solution
|
- In a face centred cubic lattice unit cell is shared equally by how man...
Text Solution
|
- In face centred cubic structure, the octahedral voids are located at :
Text Solution
|
- Crystalline solids have :
Text Solution
|
- In the unit cell of NaCl, which of the following statements are correc...
Text Solution
|
- In which of the following solids, cations occupy tetrahedral voids ?
Text Solution
|
- In which of the following type of solids, the coordination number and ...
Text Solution
|
- Which of the following statements are correct about the diamagnetic so...
Text Solution
|