Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Illustrations|47 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Practice Problem|66 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosPROBLEMS BASED UPON STRUCTURES AND REACTIONS OF ORGANIC COMPOUNDS
OP TANDON|Exercise Some Solved Problems|21 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)-MISC Examples
- Sea water is 3.5%by mass of a salt and has a density 1.04gcm^(-3) at 2...
Text Solution
|
- Molality of a solution in aqueous medium is 0.8.Calculate its more fra...
Text Solution
|
- Calculate the boiling point of a solution containing 0.61 g of benzoi...
Text Solution
|
- A very small amount of a non-volatile solute (that does not dissociate...
Text Solution
|
- A solution of a non-volatile solute in water freezes at -0.30^(@)C. Th...
Text Solution
|
- x g of non-electrolytic compound (molar mass =200) is dissolved in 1.0...
Text Solution
|
- The freezing point of a solution containing 50 cm^(3) of ethylene glyc...
Text Solution
|
- A 1.2% solution (w/v) of NaCl is isotonic with 7.2% solution(w/v) of g...
Text Solution
|
- 1.4 g of acetone dissolved in 100 g of benzene gave a solution which f...
Text Solution
|
- To 500cm^(3) of water, 3.0 xx 10^(-3) kg acetic acid is added. If 23% ...
Text Solution
|
- The osmotic pressure of a solution is 1.3 atm. The density of solution...
Text Solution
|
- (a) 10 g of a certain non-volatile solute were dissolved in 100 g wate...
Text Solution
|
- Match the boiling point with K(b) for x,y and z, if molecular weight o...
Text Solution
|
- When 1.22 g C(6)H(5)COOH is added into two solvents, the following dat...
Text Solution
|
- How much C(2)H(5)OH should be added to 1 litre H(2)O so that it will n...
Text Solution
|
- Depression in freezing point of 0.1 molal solution of HF is -0.201^(@)...
Text Solution
|
- There is KI and sucrose solution with 0.1 M concentration, if the osmo...
Text Solution
|
- 102% solution of glycerine and 2% solution of glucose are isotonic. Mo...
Text Solution
|
- Liquids A and B form ideal solution over the entire range of compositi...
Text Solution
|
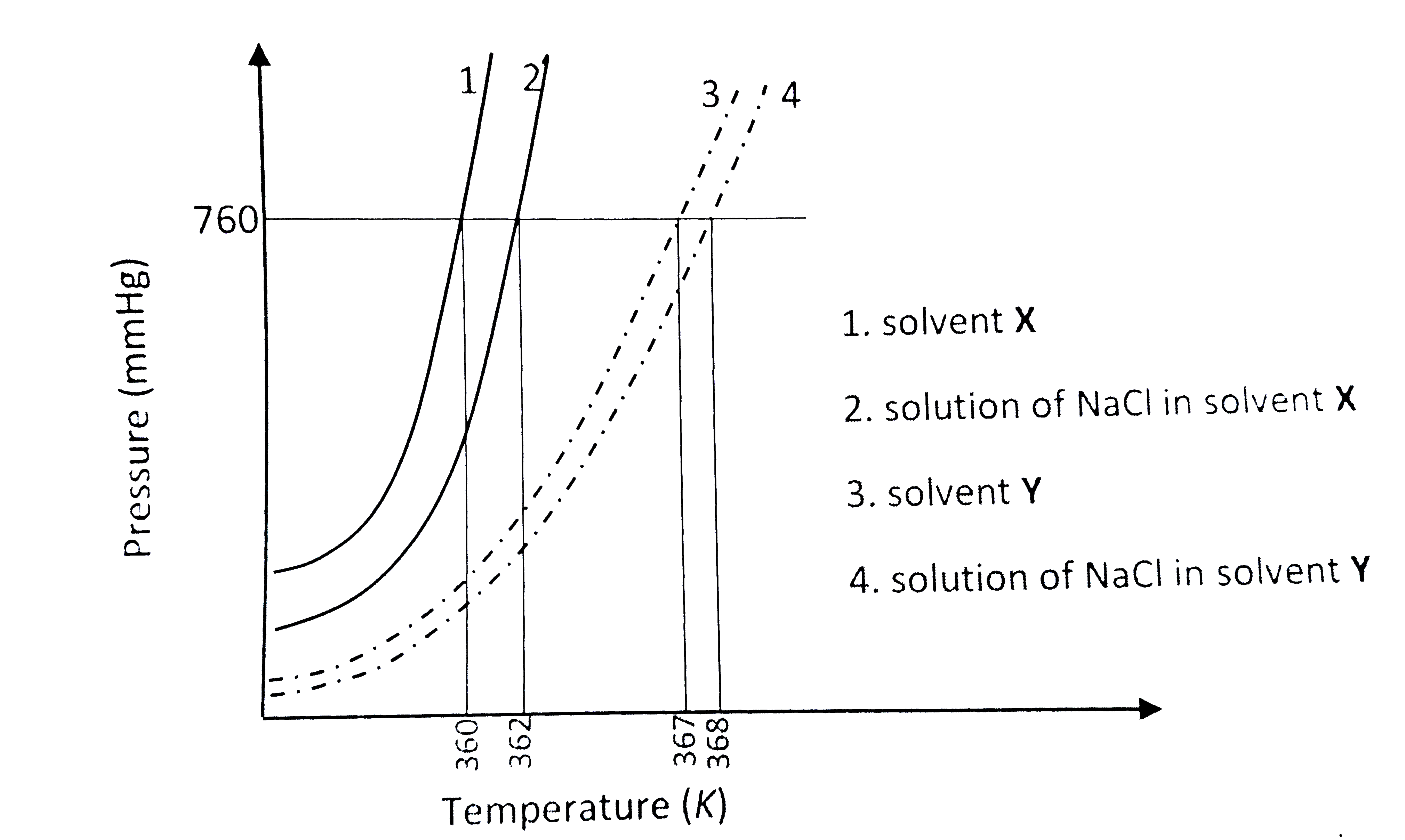
- The plot given below shows P -T curves (where P is the pressure and T ...
Text Solution
|