A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Assertion-Reason|18 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Matrix|9 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Set-2 (Level-A)|22 VideosPROBLEMS BASED UPON STRUCTURES AND REACTIONS OF ORGANIC COMPOUNDS
OP TANDON|Exercise Some Solved Problems|21 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)-Level-B
- The temperature at which molarity of pure water is equal to its molali...
Text Solution
|
- Isopiestic solution have:
Text Solution
|
- Molarity and molality of a solution of caustic soda are respectively ...
Text Solution
|
- Which of the following aqueous solution shas osmotic presseure nearest...
Text Solution
|
- Equal mass of a soute are dissolved in equal mass of two solvents A an...
Text Solution
|
- Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethan...
Text Solution
|
- Consider the following solutions: I.1 M sucrose , II. 1 M KCl III...
Text Solution
|
- The osmotic pressure of a solution depends on
Text Solution
|
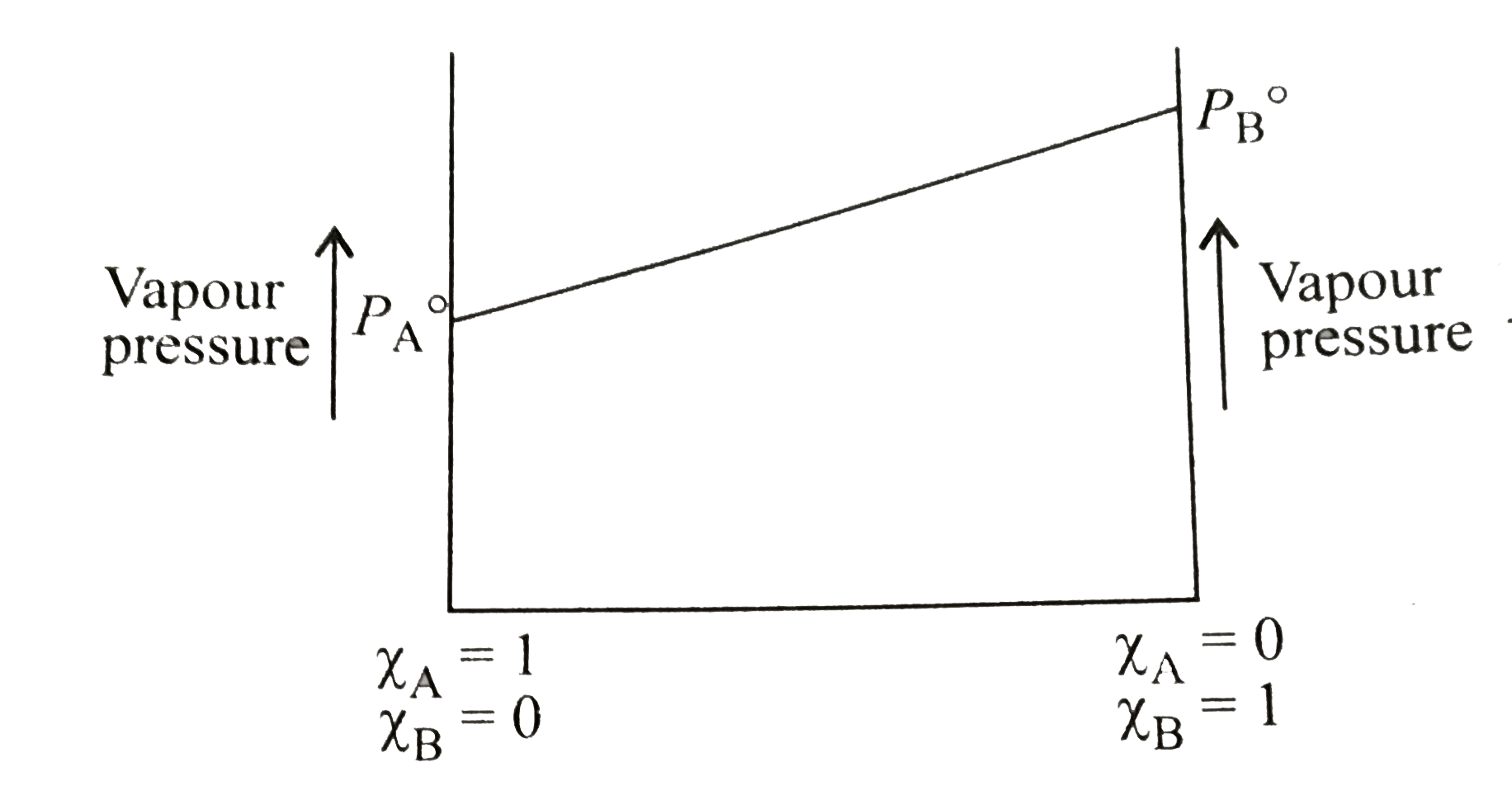
- The following is a graph plotted between the vapour pressure of two vo...
Text Solution
|
- Which of the following combinations are correct for a binary solution,...
Text Solution
|
- A solution containing 0.1g of a non-volatile organic substance P (mole...
Text Solution
|
- Consider 0.1 M solutions of two solutes X and Y. The solute X behaves...
Text Solution
|
- For a given value of degree of dissociation, which of the following ha...
Text Solution
|
- 1 mol benzene (P^(@)("benzene")=42 mm) and 2 mol toluence (P^(@)("to...
Text Solution
|
- The decrease in freezing point of an aqueous solution of a substance i...
Text Solution
|
- The vapour pressure of water at T (K) is 20 mm Hg. The following solut...
Text Solution
|
- Consider lowering of vapour pressure (Deltap), elevation in boiling po...
Text Solution
|
- Benzene and naphthalene form an ideal solution at room temperature. Fo...
Text Solution
|
- Mixture (s) showing positive deviation from Raoult's law at 35^(@)C is...
Text Solution
|
- For a solution formed by mixing liquids L and M, the vapour pressure o...
Text Solution
|