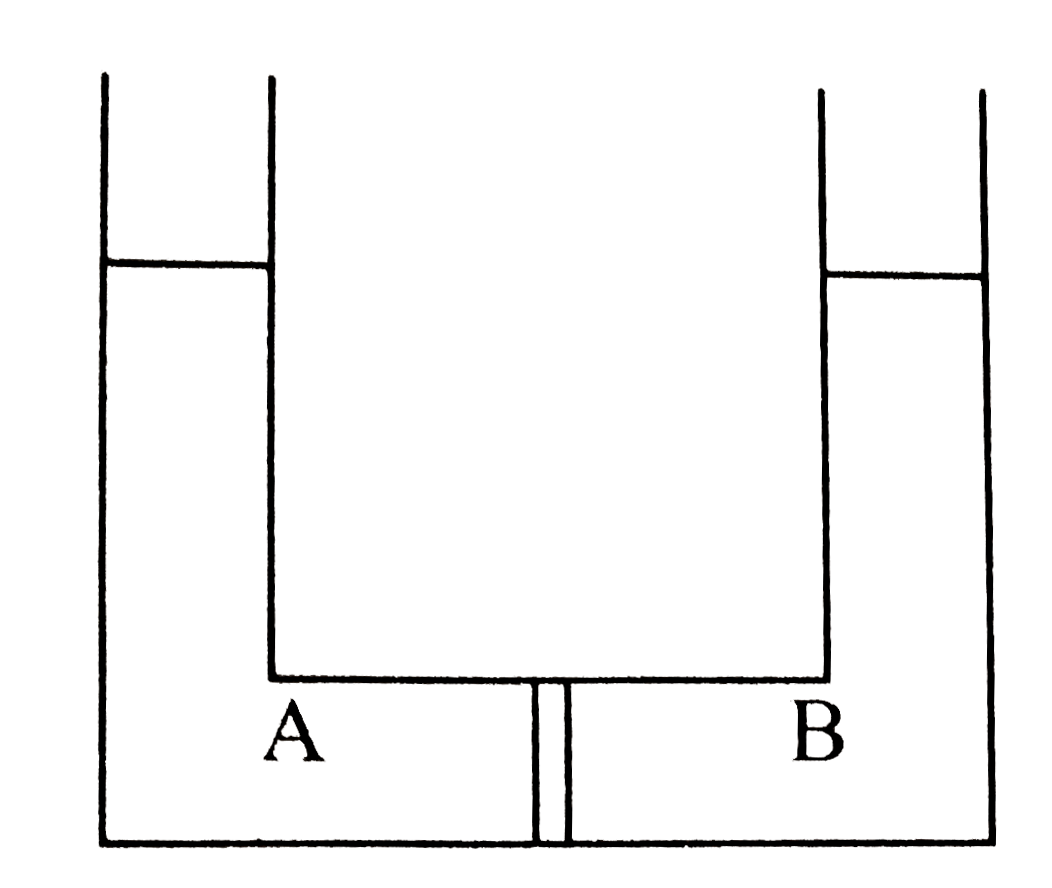
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Integer|9 VideosPROBLEMS BASED UPON STRUCTURES AND REACTIONS OF ORGANIC COMPOUNDS
OP TANDON|Exercise Some Solved Problems|21 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Self Assess,ent|28 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)-Comprehension
- The osomotic pressure pi depends on the molar concentration of the sol...
Text Solution
|
- The osomotic pressure pi depends on the molar concentration of the sol...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The solution which boil at constant temperature like a pure liquid and...
Text Solution
|
- The solution which boil at constant temperature like a pure liquid and...
Text Solution
|
- The solution which boil at constant temperature like a pure liquid and...
Text Solution
|
- The solution which boil at constant temperature like a pure liquid and...
Text Solution
|
- The solution which boil at constant temperature like a pure liquid and...
Text Solution
|
- Properties such as boiling point, freezing point and vapour pressure o...
Text Solution
|
- Properties such as boiling point, freezing point and vapour pressure o...
Text Solution
|
- Properties such as boiling point, freezing point and vapour pressure o...
Text Solution
|