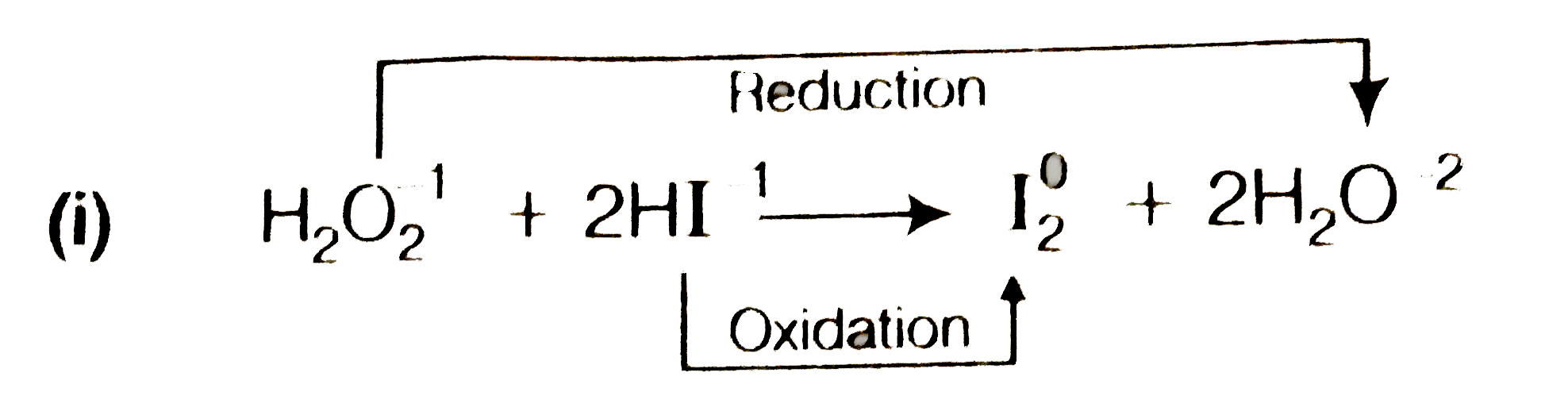A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN
NCERT EXEMPLAR|Exercise SHORT ANSWER TYPE QUESTIONS|35 VideosHYDROGEN
NCERT EXEMPLAR|Exercise MATCHING THE COLUMNS|4 VideosHYDROCARBONS
NCERT EXEMPLAR|Exercise Long Answer Type Questions|4 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT EXEMPLAR|Exercise Long Answer type question|6 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-HYDROGEN-LONG ANSWER TYPE QUESTIONS
- Cosider the reactions (i) H(2)O(2) + 2HI to I(2) + 2H(2)O (ii...
Text Solution
|
- Atomic hydrogen combaines with almost all elements but molecular ...
Text Solution
|
- How can D(2)O(2) prepared from water ? Mention the physical propert...
Text Solution
|
- How will you concentrate H(2)O(2) ? Show difference between struc...
Text Solution
|
- Give a method for the manufacture of hydrogen peroxide and explain ...
Text Solution
|
- (i) What mass of hydrogen peroxide will be present in 2 L of a 5 m...
Text Solution
|
- A colourless liquid 'A' contains H and O elements only. It decompose...
Text Solution
|
- An ionic hydride of an alkali metal has significant covalent chara...
Text Solution
|
- Sodium form a crystallic ionic solid with dihdrogen . The solid ...
Text Solution
|