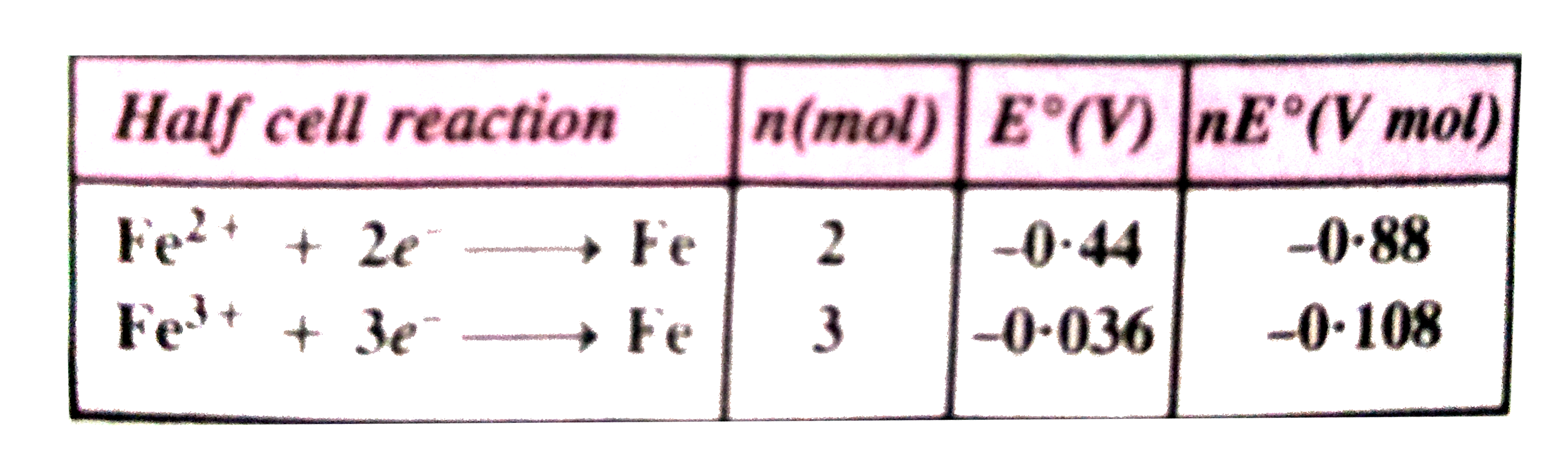A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
DINESH PUBLICATION|Exercise Example|74 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise IN-TEXT QUESTIONS|15 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ASSERTION AND REASON|20 VideosD-AND -F BLOCK ELEMENTS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosETHERS
DINESH PUBLICATION|Exercise (MCQs)|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ELECTROCHEMISTRY-ULTIMATE PREPARATORY PACKAGE
- Cu^(2+)(aq.) is unstable in solution and under goes simultaneous oxida...
Text Solution
|
- Cell reactiomn is spontaneous when
Text Solution
|
- What is the potential of a cell containing hydrogen electrodes, the ne...
Text Solution
|
- The standard electrode potentials (E^(@)) " for " Ocl^(-)//Cl^(-) " an...
Text Solution
|
- A current of 2.0A passed for 5 hours through a molten metal salt depos...
Text Solution
|
- Given standard electode potenitals Fe^(2+) + 2e^(-) rarr Fe, E^@ =-...
Text Solution
|
- An electrochemical cell is shown below Pt, H(2)(1 "atm")|HCl(0.1 M)|C...
Text Solution
|
- The reduction potential of hydrogen half cell will be negative if :
Text Solution
|
- In an electrolytic cell, one litre of a 1 M aqueous solution of MnO(4)...
Text Solution
|
- On electrolysis, which of the following does not give out hydrogen?
Text Solution
|
- The specific conductances of four electrolytes in ohm^(-1)cm^(-1) are ...
Text Solution
|
- The value of (E(H(2)O)//H(2)^(@)) (1atm) Pt at 298K would b e
Text Solution
|