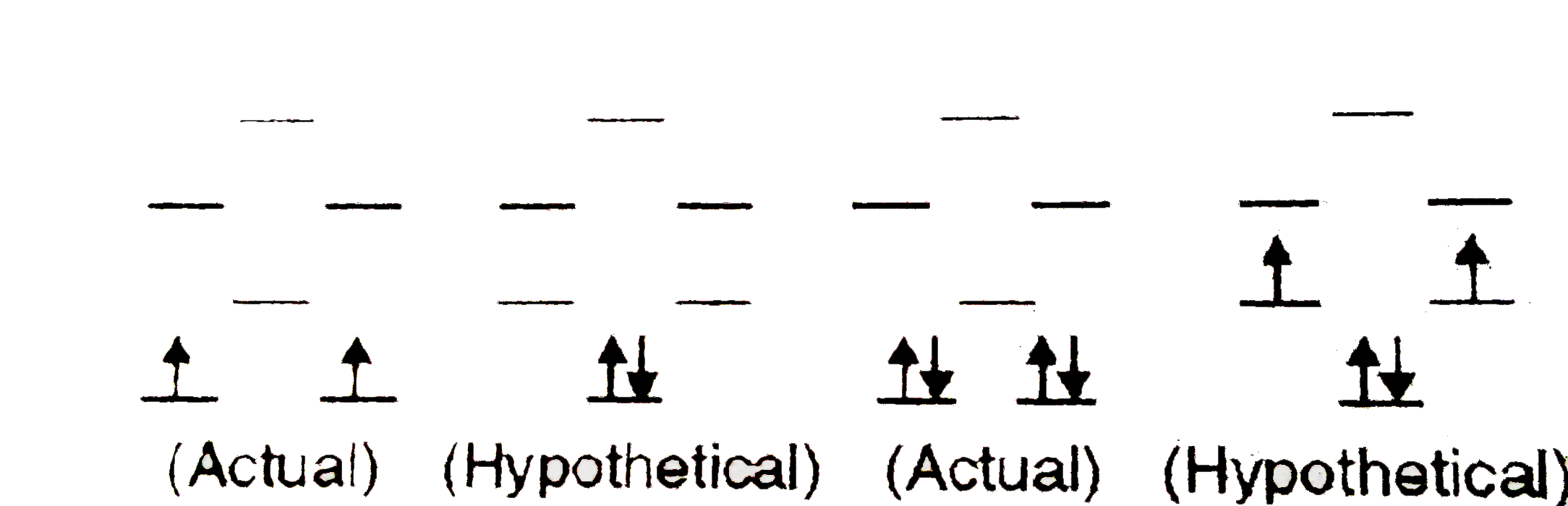A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Brain Teaser-1|40 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Brain Teaser-2|40 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Reason Assertion|37 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Ultimate Preparatory Package
- Consider the b.pr. of Br2 and ICl. The b.pt. of Br2
Text Solution
|
- The decreasing order of polarity of the bonds in NH3, PH3, AsH3 and Sb...
Text Solution
|
- The substance with highest boiling point out of the following H2, He, ...
Text Solution
|
- At 300 K and 1.00 atm. pressure, the density of gaseous HF is 3.17 gL^...
Text Solution
|
- The suggested molecular orbital electronic configuration of Co is : KK...
Text Solution
|
- In N2H4 (hydrazine) both the nitrogen atoms are
Text Solution
|
- The expected shape of Br3^- ion is
Text Solution
|
- The shapes of molecules of C Cl4, XeF4 and SF4 are
Text Solution
|
- The shapes of molecules of BF3, NH3 and ClF3 are
Text Solution
|
- Non-bonding orbitals have
Text Solution
|
- Suppose energy level diagram used for O2 F2 etc. is used for all homon...
Text Solution
|
- Arrange the following in the increasing order of melting points (a)N...
Text Solution
|
- The number of lobes in delta and delta^** formed by the overlap of two...
Text Solution
|
- Ground state He2 does not exist. An electronic excited state of He2
Text Solution
|
- The energy of sigma(2s), is greater than that of sigma(1s)^** orbital ...
Text Solution
|
- A molecule of chloral hydrate contains two -OH groups attached to a si...
Text Solution
|
- The bond length in O2^+, O2 ,O2^- and O2^(2-) follows the order :
Text Solution
|
- The order of energies of following combination (a)2HHe (b)H2+He2 (c ...
Text Solution
|
- Which of the following is paramagnetic O2^(2-) and BN ?
Text Solution
|
- Pick out the incorrect statement
Text Solution
|