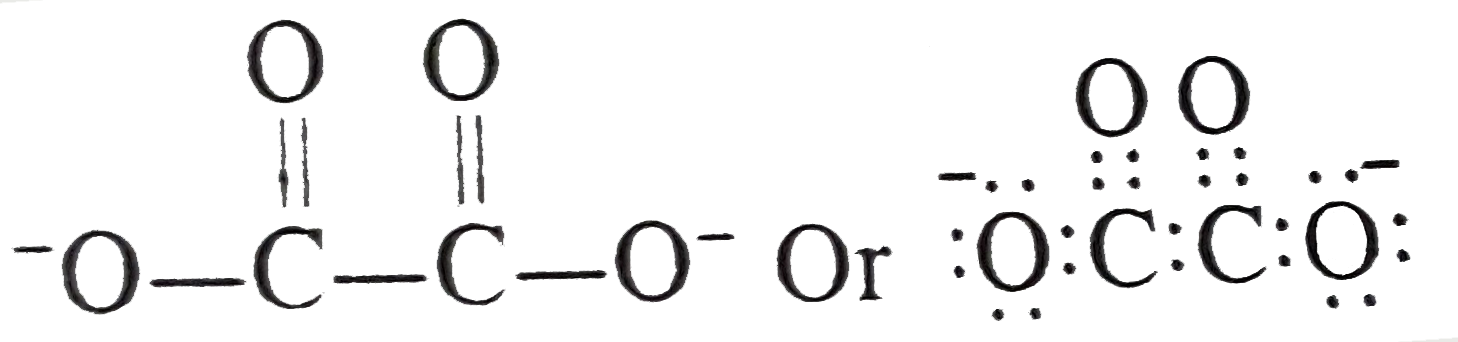A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Brain Teaser-1|40 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCHEMICAL KINETICS
DINESH PUBLICATION|Exercise Additional Numerical Problems For Practice|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Brain Teaser-2
- The correct increasing bond angle among BF3 , PF3 and ClF3 follows th...
Text Solution
|
- The correct order of increasing electropositive character among Cu ,Fe...
Text Solution
|
- The size of ionic species is correctly given in the order
Text Solution
|
- The electronegativity difference between two atoms A and B is 2, then ...
Text Solution
|
- There are two nodes in the radial probability distribution curve for t...
Text Solution
|
- The bond angle in H2O (for H-S-H) is
Text Solution
|
- If the ionic radii of each K^(+) and F^(-) are 1.34Å, then tha atomic ...
Text Solution
|
- One among the following is the incrorrect order of increasing ionisati...
Text Solution
|
- Which of the following is false ?
Text Solution
|
- The d-orbitals involved in dsp^2 hybridisation is
Text Solution
|
- A 5.82 g silver coin is dissolved in nitric acid. When sodium chloride...
Text Solution
|
- Which of the following linear combinations of atomic orbitals is incor...
Text Solution
|
- In one among the following molecules the state of hybridisation of the...
Text Solution
|
- The state of hybridisation of sulphur in SO2 is same as that of sulph...
Text Solution
|
- The magnetic character of oxygen molecule is he same as that of one of...
Text Solution
|
- The density of the nucleus is
Text Solution
|
- Azimuthal Quantum number was given by
Text Solution
|
- Diatomic molecule has a dipole moment of 1.2D If its bond 1.0 Å what f...
Text Solution
|
- How many electrons are used in bonding the Lewis structure of C2O4^(2-...
Text Solution
|
- The energy required to stop ejection of electrons from a Cu plate is 0...
Text Solution
|