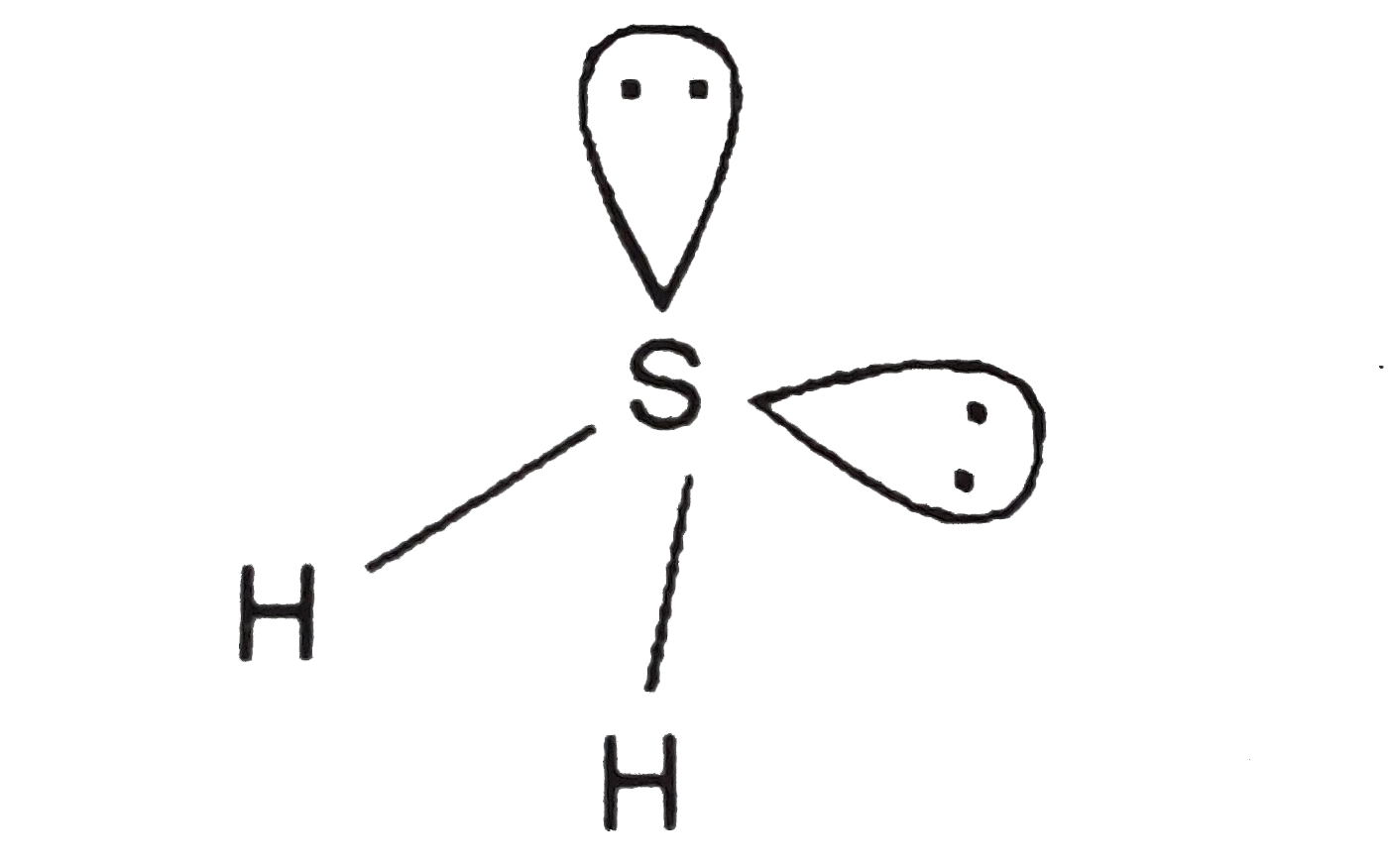
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE OXYGEN FAMILY
DINESH PUBLICATION|Exercise MATRIX MATCH|2 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise INTEGER|4 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise REVISION|143 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 VideosTHE P-BLOCK ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Choice Questions (McQs)|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE OXYGEN FAMILY-Selected Straight objective
- The first ionisation potential in electron volts of nitrogen and oxyge...
Text Solution
|
- There is no S-S bond in
Text Solution
|
- The oxidation states of the most electronegative elements in the produ...
Text Solution
|
- Which compound acts as an oxidising as well as reducing agent?
Text Solution
|
- A substance on treatment with dilute H(2)SO(4) liberates a colourless ...
Text Solution
|
- Hydrolysis of one mole of peroxodisulphuric acid produces
Text Solution
|
- Sodium thiosulphate is prepared by
Text Solution
|
- Which one of the following compounds has sp^(2) hybridization?
Text Solution
|
- Sodium nitrate decomposes above 800^(@)C to give :
Text Solution
|
- The oxidation number of sulphur in S(8), S(2)F(2) and H(2)S respective...
Text Solution
|
- The geometry of H(2)S and its dipole moment are :
Text Solution
|
- Amongst H2O, H2S, H2 Se and H2 Te, the one with the highest boiling po...
Text Solution
|
- The number of S-S bonds in sulphur trioxide trimer (S(3)O(9)) is
Text Solution
|
- Identify the correct order of acidic strength of CO(2),CuO, CaO and H(...
Text Solution
|
- [X]+H(2)SO(4) rarr [Y] a colourless gas with irritating smell [Y] + K(...
Text Solution
|
- The acid having O - O bond is
Text Solution
|
- Which of the following molecular species has unpaired electrons(s) ? .
Text Solution
|
- Which of the following statements is true ?
Text Solution
|
- Which of the following is the most boric oxide
Text Solution
|
- Angular shape of ozone molecule consists of
Text Solution
|