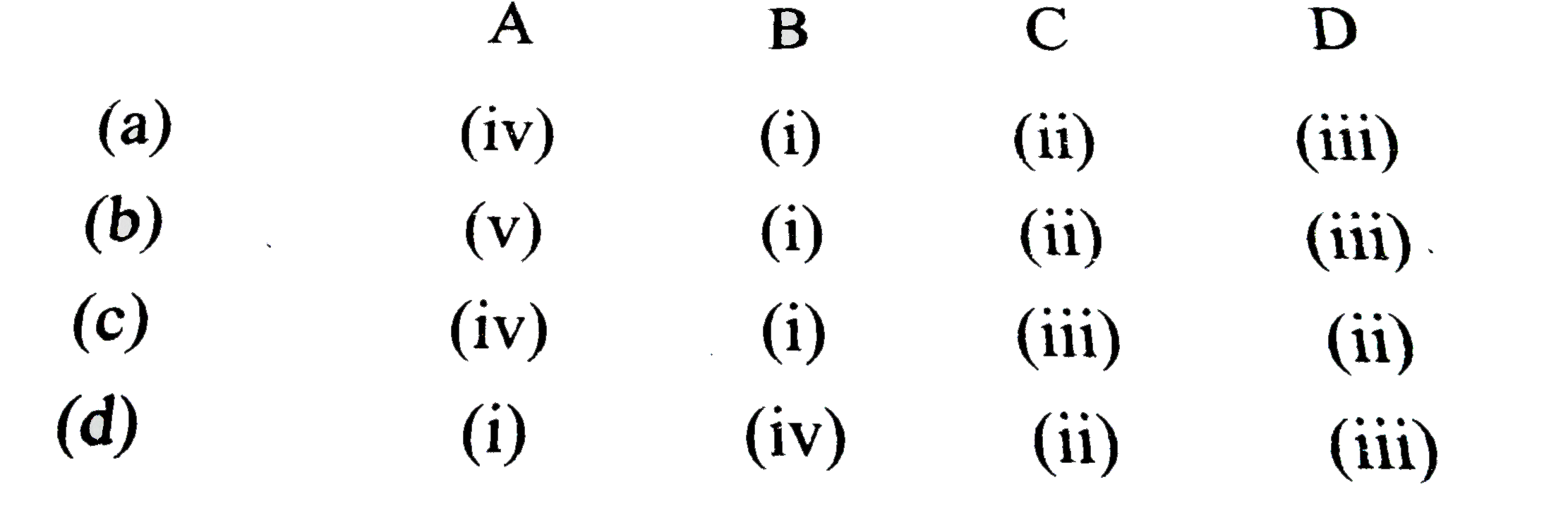Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise OBJECTIVE B|31 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise INTEGER ANSWER TYPE QUESTIONS|5 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise Practical Problems|23 VideosSATURATED ALIPHATIC HYDROCARBONS
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|7 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|10 Videos
OP TANDON-STOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )-OBJECTIVE QUESTIONS (LEVEL-A)
- In the reaction: 2Al((s))+6HCl((aq.))rarr2Al((aq.))^(3+)+6Cl((aq.))^...
Text Solution
|
- Calculate the weight of iron which will be converted into its oxide by...
Text Solution
|
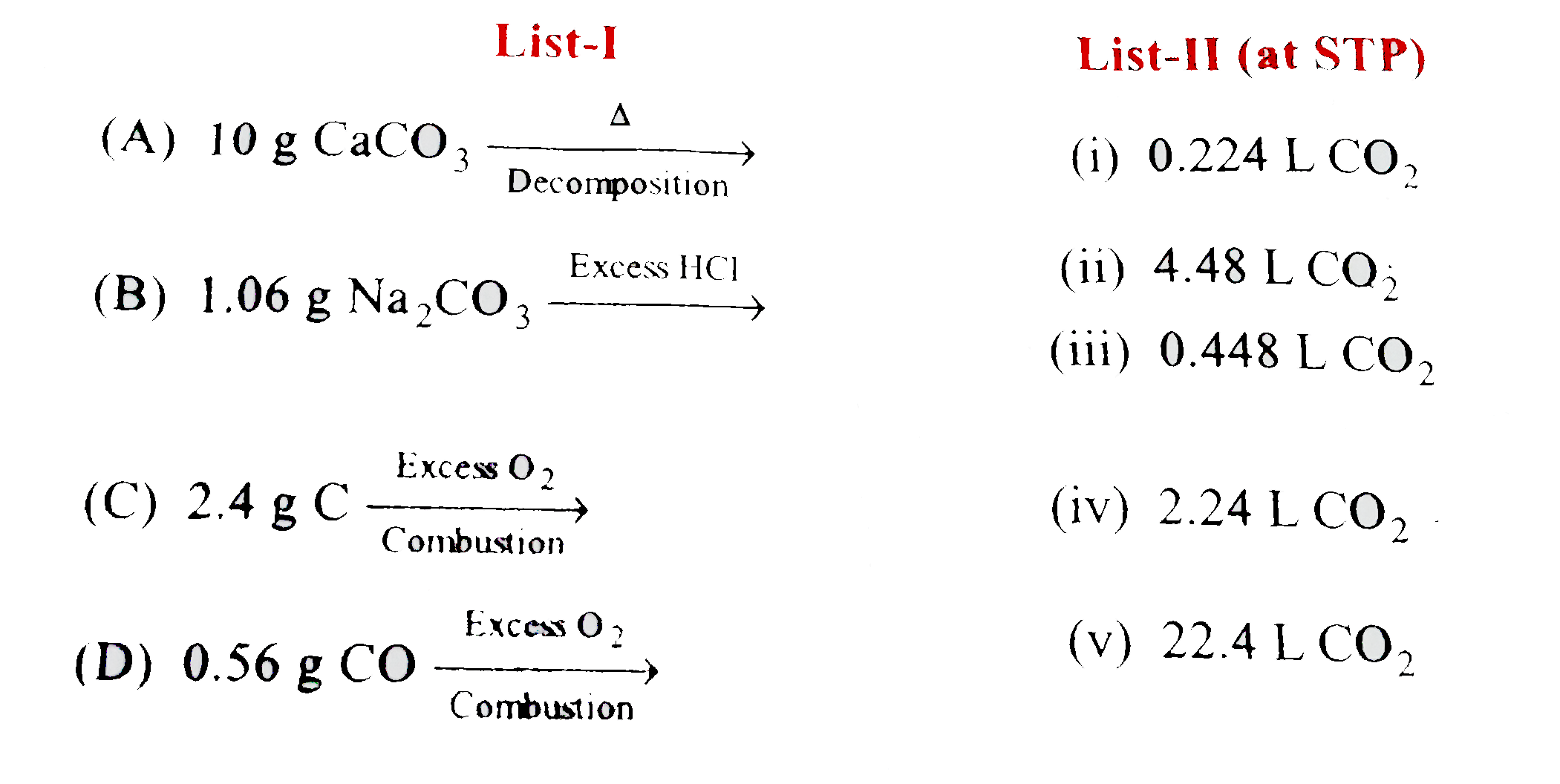
- Match the following
Text Solution
|
- Fe^(2+)toFe^(3+)+e^(-), MnO(4)^(-)+5e^(-)toMn^(2+), the ratio of stoic...
Text Solution
|
- What volume of oxygen gas (O(2)) measured of 0^(@)C and 1 am needed t...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- 1 mole of methylamine on reaction with nitrous acid gives at NTP:
Text Solution
|
- The value of 'n' in the reaction underset(1"mol")Cr(2)O(7)^(2-)+14H...
Text Solution
|
- In the complex with formula MCl(3).4H(2)O the co-ordination number of ...
Text Solution
|
- 1.5g CdCl(2) was formed to contain 0.9g Cd. Calculate MO, the atomic w...
Text Solution
|
- 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and...
Text Solution
|
- In an experiment, 4g of M(2)O(x) oxide was reduced to 2.8g of the met...
Text Solution
|
- A vessel fitted with a weightless, frictionless piston of 0.025m^(2) a...
Text Solution
|
- The reaction of calcium with water is represented by the equation Ca...
Text Solution
|
- What volume of hydrogen will be liberated at NTP by the reaction of Zn...
Text Solution
|
- Calculate the mass of oxygen obtained by complete decomposition of 10k...
Text Solution
|
- The molecular formula of a commercial resin used for exchanging ions i...
Text Solution
|
- A water sample contains fMgSO What is . 68 mofCaSO4and60ppmo 4· . Mg(H...
Text Solution
|
- At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air...
Text Solution
|
- 1 gram of carbonate (M(2)CO(3)) on treatment with excess HCl produces ...
Text Solution
|