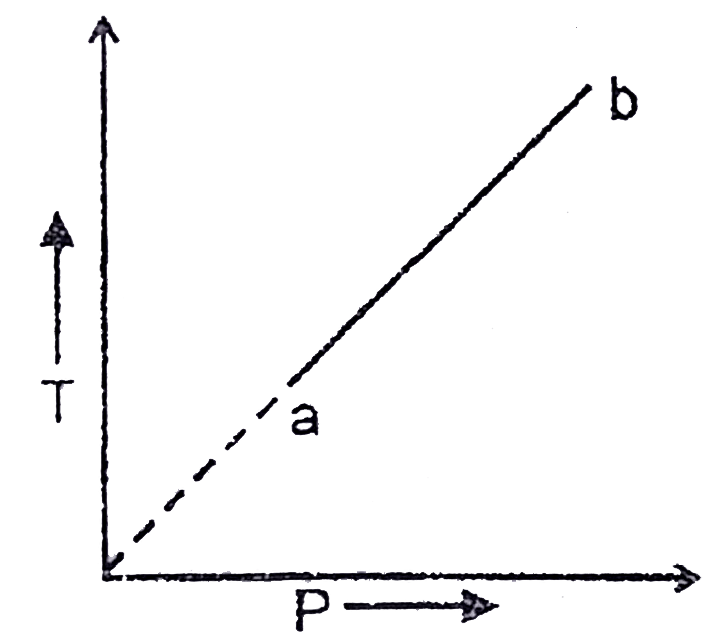
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMICS-Exercise -1 Part -II Only option correct type
- The work done in ergs for the reversible expansion of one mole of an i...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- An ideal gas change from state a to state b as shown in Fig. what is t...
Text Solution
|
- In given figure, let Delta W and Delta W(2) be the work done by the ga...
Text Solution
|
- Freezing up liquid in a system then:
Text Solution
|
- A piece of zinc at a temperature of 20^(@)C weighing 65.38 g is droppe...
Text Solution
|
- Which has maximum internal energy at 290K ?
Text Solution
|
- Let Delta U(1) and Delta U(2) be the changes in internal energy of an ...
Text Solution
|
- The process Delta U=0, for an ideal gas can be best represented in the...
Text Solution
|
- For 2 mole of an ideal gas , the relation between C(p) & C(v) (non-mol...
Text Solution
|
- When an ideal gas is heated at constant pressure, the fraction of the ...
Text Solution
|
- Supposing the distance between the atoms of a diatomic gas to be const...
Text Solution
|
- The following sets of volume of C(v) and C(p) of an ideal gas have bee...
Text Solution
|
- A system absorb 600 J of heat and work equivalent to 300 J on its surr...
Text Solution
|
- A sample of liquid in a thermally insulated constant ( a calorimetre...
Text Solution
|
- In an isochoric process the increase in internal energy is
Text Solution
|
- Ideal gas is taken through the process shown in the figure :
Text Solution
|
- If heat is supplied to an ideal gas in an isothermal process.
Text Solution
|
- In an isothermal expansion of an ideal gas. Select wrong statement:
Text Solution
|
- A system undergoes a process which absorbed 5 KJ of heat and undergoin...
Text Solution
|