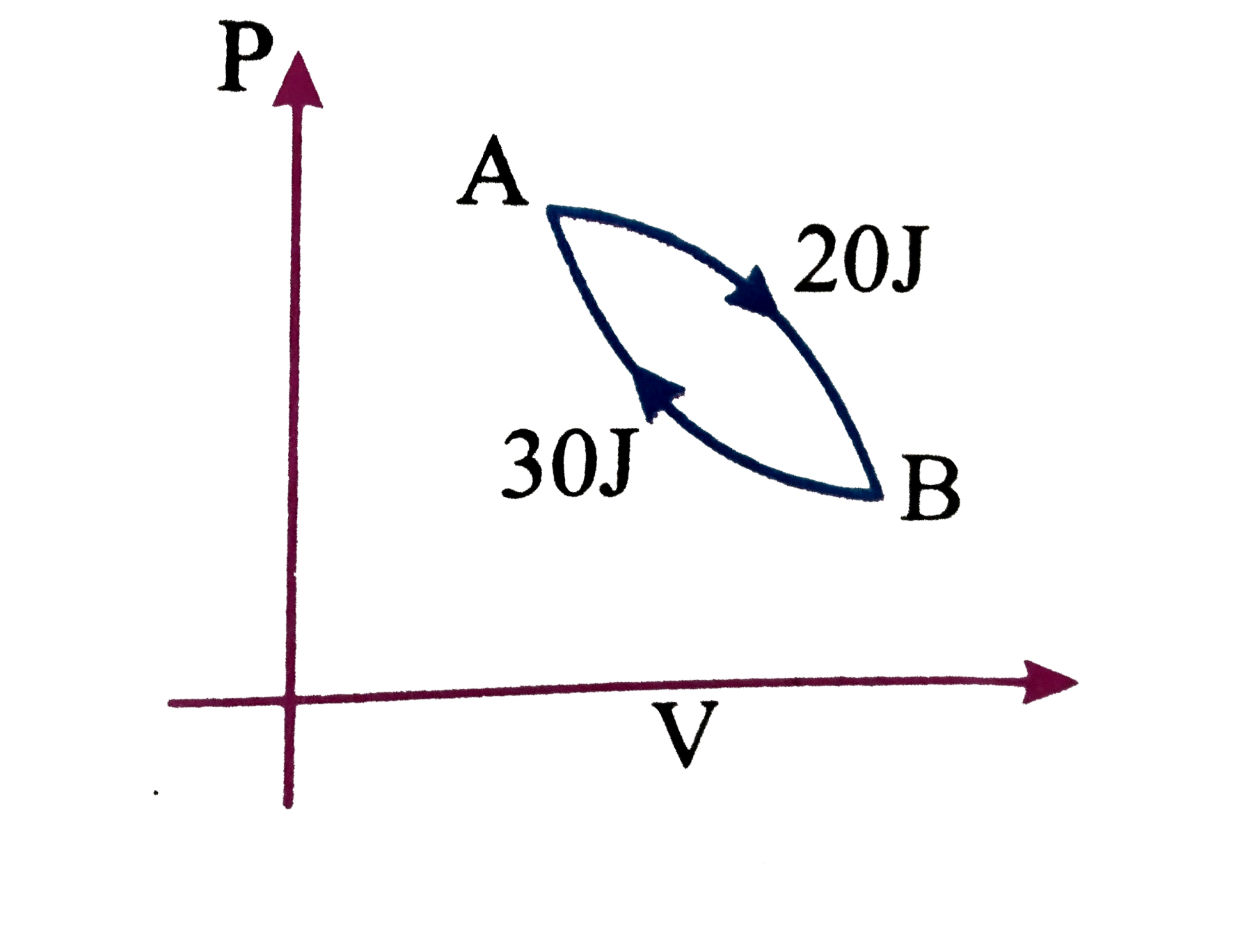
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMICS-Exercise -1 Part -II Only option correct type
- A balloon of 1 L volume is fill with O(2) gas, kept in a spaceship at...
Text Solution
|
- 1 mole of an ideal diatomic gas undergoes a reversible polytropic proc...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adiabatically...
Text Solution
|
- The molar heat capacity for the process shown in fig. is
Text Solution
|
- In figure, A and B are two adiabatic curves for two different gases. T...
Text Solution
|
- If w(1).w(2),w(3) and w(4) are work done in isothermal, adiabatic, iso...
Text Solution
|
- One mole of non- ideal gas undergoes a change of state (1.0 atm, 3.0L,...
Text Solution
|
- A system containing real gas changes it's state from state -1 to state...
Text Solution
|
- For the isothernmal expansion of an ideal gas
Text Solution
|
- A container of volume 2L is seperated into equal compartments. In one ...
Text Solution
|
- A vessel contains 100 litres of a liquid X. Heat is supplied to the li...
Text Solution
|
- H(2)O((s))rarrH(2)O((l)) This phase transition is carried out at con...
Text Solution
|
- At 1 atm pressure freezing of n mole of water liquid (0^(@)C) then hea...
Text Solution
|
- The free energy change for a reversible reaction at equilibrium is:
Text Solution
|
- Delta H^(@) for the reaction X((g))+Y((g))hArrZ((g)) is -4.6 kcal, the...
Text Solution
|
- Determine which of the following reactions at constant pressure repres...
Text Solution
|
- Consider the reaction at 300 K H(2)(g)+Cl(2)(g)rarr2HCl(g), " "Del...
Text Solution
|
- If DeltaH is the enthalpy change and DeltaU the change in internal ene...
Text Solution
|
- Which plot represent an exothermic reaction ?
Text Solution
|
- For which of the following change Delta H!=Delta U?
Text Solution
|