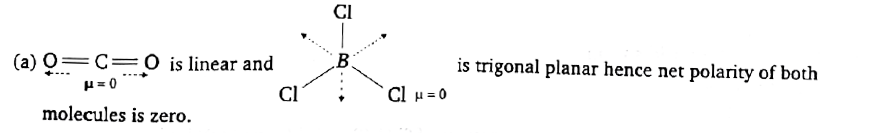A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 1)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 2)|6 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-Level 3 (Passive 11)
- Correct set of species with zero dipole moment is : (i) CO(2) " "...
Text Solution
|
- Consider the following elements with their period number and valence e...
Text Solution
|
- Consider the following elements with their period number and valence e...
Text Solution
|
- Consider the following elements with their period number and valence e...
Text Solution
|
- Consider the following elements with their period number and valence e...
Text Solution
|
- Consider the following elements with their period number and valence e...
Text Solution
|
- Consider the following elements with their period number and valence e...
Text Solution
|