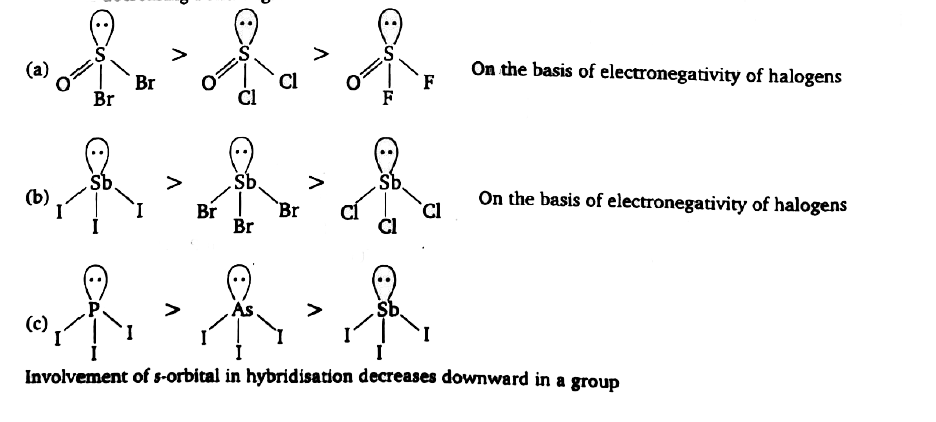
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 3)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 4)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|54 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-Level 2
- Choose the correct option for the collowing molecule in view of chemic...
Text Solution
|
- Which of the following statement is correct about I(3)^(+) and I(3)^(-...
Text Solution
|
- In which of the following molecular shape d(z^(2)) orbital must not be...
Text Solution
|
- The correct statement regarding SO(2) molecule is :
Text Solution
|
- A molecule XY(2) contains two sigma bonds two pi bond and one lone pai...
Text Solution
|
- In which of the following pairs, both the species have the same hybrid...
Text Solution
|
- Which of the following possess two lone pair of electrons on the centr...
Text Solution
|
- Select pair of compounds in which both have different hybridization bu...
Text Solution
|
- The species having no ppi-ppi bond but its bond order equal to that of...
Text Solution
|
- Which of the following fact is directly explained by the statement oxy...
Text Solution
|
- Which of the following compound has maximum "C-C" single bond length ?
Text Solution
|
- If two different non-axial d-orbitals having 'xz' nodal plane form pi-...
Text Solution
|
- Assuming pure 2s and 2p orbitals of carbon are used in forming CH(4) m...
Text Solution
|
- Which of the following is correct order of sigma - bond strength ? ...
Text Solution
|
- Which of the following statements in incorrect for sigma and pi -bonds...
Text Solution
|
- Assuming the bond direction to the z-axis, which of the overlapping of...
Text Solution
|
- Which of the following orbital can not form pi as well as delta-Bond ?
Text Solution
|
- Incorrect statement is :
Text Solution
|
- Which of the following set contains species having same angle around t...
Text Solution
|
- Which of the following compound has the smallest (X-A-X) bond angle in...
Text Solution
|