A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 12)|4 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise ONE OR MORE ANSWERS IS / ARE CORRECT|48 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 9)|6 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-Level 3 (Passive 10)
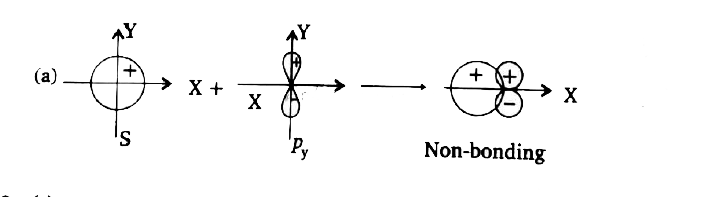
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|