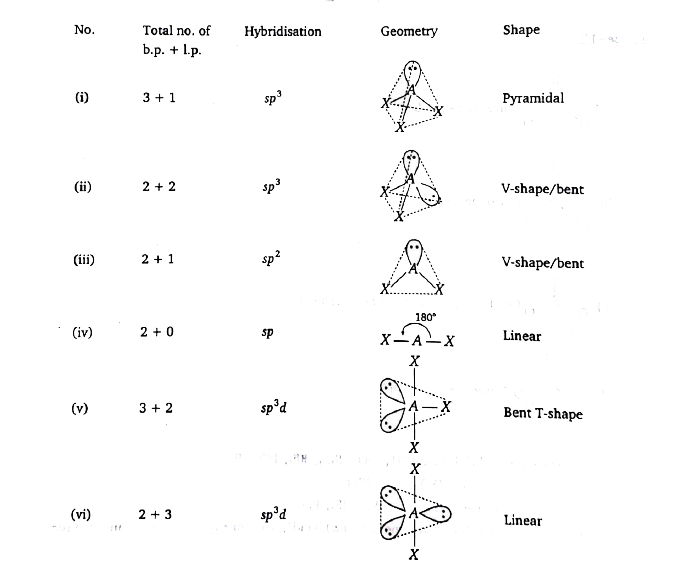A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise MATCH THE COLUMN|25 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|24 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 12)|4 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-ONE OR MORE ANSWERS IS / ARE CORRECT
- Which of the following species does / do not exist ?
Text Solution
|
- Which of the following species is /are superoctet molecule ?
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Which of the following species is /are capable of forming a coordinate...
Text Solution
|
- Ionic compounds in geneal do not possess :
Text Solution
|
- Correct statbility order of metal cation is /are :
Text Solution
|
- Consider the following molecule : underset((1))(H(2)C)=underset((2))...
Text Solution
|
- Consider the following two molecules and according to the given inform...
Text Solution
|
- Which of the following statements are correct about sulphur hexafluori...
Text Solution
|
- If AB(4)^(n) types species are tetrahedral, then which of the followin...
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- Which of the following combination of bond pair (b.p.) and lone pair (...
Text Solution
|
- Select the true statement(s) among the following :
Text Solution
|
- p(y)-orbital can not form pi -bond by lateral overlap with :
Text Solution
|
- Which of the following orbital (s) cannot form delta-bond ?
Text Solution
|
- Select correct statement(s) regarding sigma and pi bonds :
Text Solution
|
- Which of the following statements is / are correct ?
Text Solution
|
- Consider the following three orbitals : Correct statement(s) rega...
Text Solution
|
- Which of the following combination of orbitals do / does not form bon...
Text Solution
|
- Consider the following atomic orbitals : Which of the following s...
Text Solution
|