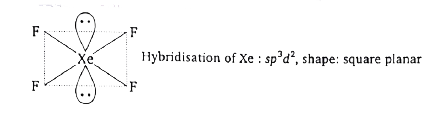A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|54 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 2|62 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise MATCH THE COLUMN|25 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-ASSERTION-REASON TYPE QUESTIONS
- Assertion : All diatomic molecules with polar bond have dipole moment....
Text Solution
|
- Assertion : Water is a good solvent for ionic compounds but poor one...
Text Solution
|
- Assertion : Xe-atom in XeF(2) assumes sp-hybrid state. Reason : XeF(...
Text Solution
|
- Assertion : The atoms in a covalent molecule are said to share el...
Text Solution
|
- Assertion : C Cl(4) is a non-polar molecule. Reason : C Cl(4) has p...
Text Solution
|
- Assertion : Geometry of IC l(3) is tetrahedral. Reason : Its shape i...
Text Solution
|
- Assertion : The covalency of carbon is four in excited state. Reason...
Text Solution
|
- Assertion : The shape of XeF(4) is square- planar. Reason : In an oc...
Text Solution
|
- Assertion : Multiple bond between two bonded atoms can have more than ...
Text Solution
|
- Assertion : 2^(nd) period elements do not involve in excitation of ele...
Text Solution
|
- Assertion : In SO(3) molecule bond dissociation energy of all S=O bond...
Text Solution
|
- Assertion : PH(4)^(+) ion is having tetrahedron geometry. Reason : P...
Text Solution
|
- Assertion : All diatomic molecules with polar bond have dipole moment....
Text Solution
|
- Assertion : Water is a good solvent for ionic compounds but poor one...
Text Solution
|
- Assertion : Xe-atom in XeF(2) assumes sp-hybrid state. Reason : XeF(...
Text Solution
|
- Assertion : The atoms in a covalent molecule are said to share el...
Text Solution
|
- Assertion : C Cl(4) is a non-polar molecule. Reason : C Cl(4) has p...
Text Solution
|
- Assertion : Geometry of IC l(3) is tetrahedral. Reason : Its shape i...
Text Solution
|
- Assertion : The covalency of carbon is four in excited state. Reason...
Text Solution
|
- Assertion : The shape of XeF(4) is square- planar. Reason : In an oc...
Text Solution
|