Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
CHHAYA PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS|12 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise ENTRANCE QUESTION|20 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise SOLVED WBCHSE SCANNNER|20 VideosCLASSIFICATION OF ELEMENTS & PERIODICITY IN PROPERTIES
CHHAYA PUBLICATION|Exercise PRACTICE SET|12 Videoss-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET|16 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-REDOX REACTIONS -SOLVED NCERT EXERCISE
- Justify the following reactions are redox reactions : 4NH(3)(g)+5O(2...
Text Solution
|
- Fluroine reacts with ice and results in the change : H(2)O+F(2)(g)toHF...
Text Solution
|
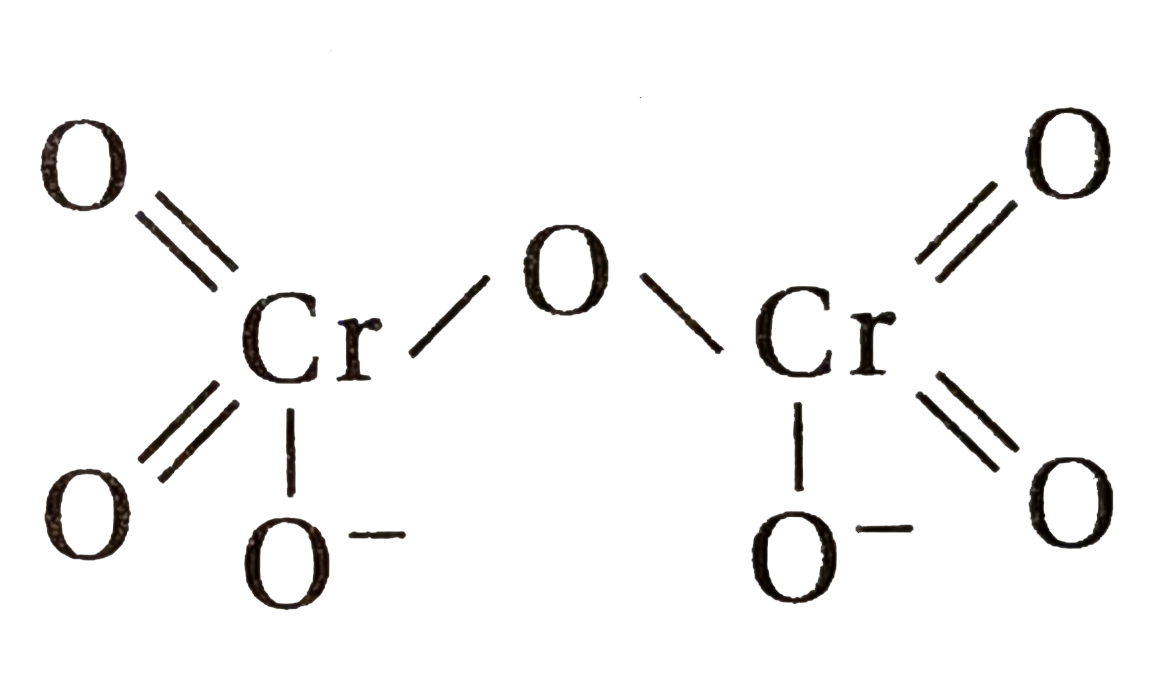
- Calculate the oxidation number of sulphur, chromium and nitrogen in H(...
Text Solution
|
- Write fromula for the following compounds: Mercury (II) chloride
Text Solution
|
- Write fromula for the following compounds: Nickel (II) sulphate
Text Solution
|
- Write fromula for the following compounds: Tin(IV) oxide
Text Solution
|
- Write formula for the following compounds: Thallium(I) sulphate
Text Solution
|
- Write fromula for the following compounds: Iron (III) sulphate
Text Solution
|
- Write fromula for the following compounds: Chromium (III) oxide
Text Solution
|
- Suggest a list of the substance where carbon can exhibit oxidation sta...
Text Solution
|
- While sulphur dioxide and hydrogen peroxide can act as oxidising as we...
Text Solution
|
- consider the reactions: 6CO(2)(g)+6H(2)O(l)toC(6)H(12)O(6)(aq)+6O(2)...
Text Solution
|
- consider the reactions: 6CO(2)(g)+6H(2)O(l)toC(6)H(12)O(6)(aq)+6O(2)...
Text Solution
|
- consider the reactions: 6CO(2)(g)+6H(2)O(l)toC(6)H(12)O(6)(aq)+6O(2)...
Text Solution
|
- consider the reactions: 6CO(2)(g)+6H(2)O(l)toC(6)H(12)O(6)(aq)+6O(2)...
Text Solution
|
- consider the reactions: 6CO(2)(g)+6H(2)O(l)toC(6)H(12)O(6)(aq)+6O(2)...
Text Solution
|
- consider the reactions: 6CO(2)(g)+6H(2)O(l)toC(6)H(12)O(6)(aq)+6O(2)...
Text Solution
|
- The compound AgF(2) is an unstable compound, However ,if formed the co...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- How do you count the following obeservation ?
Text Solution
|