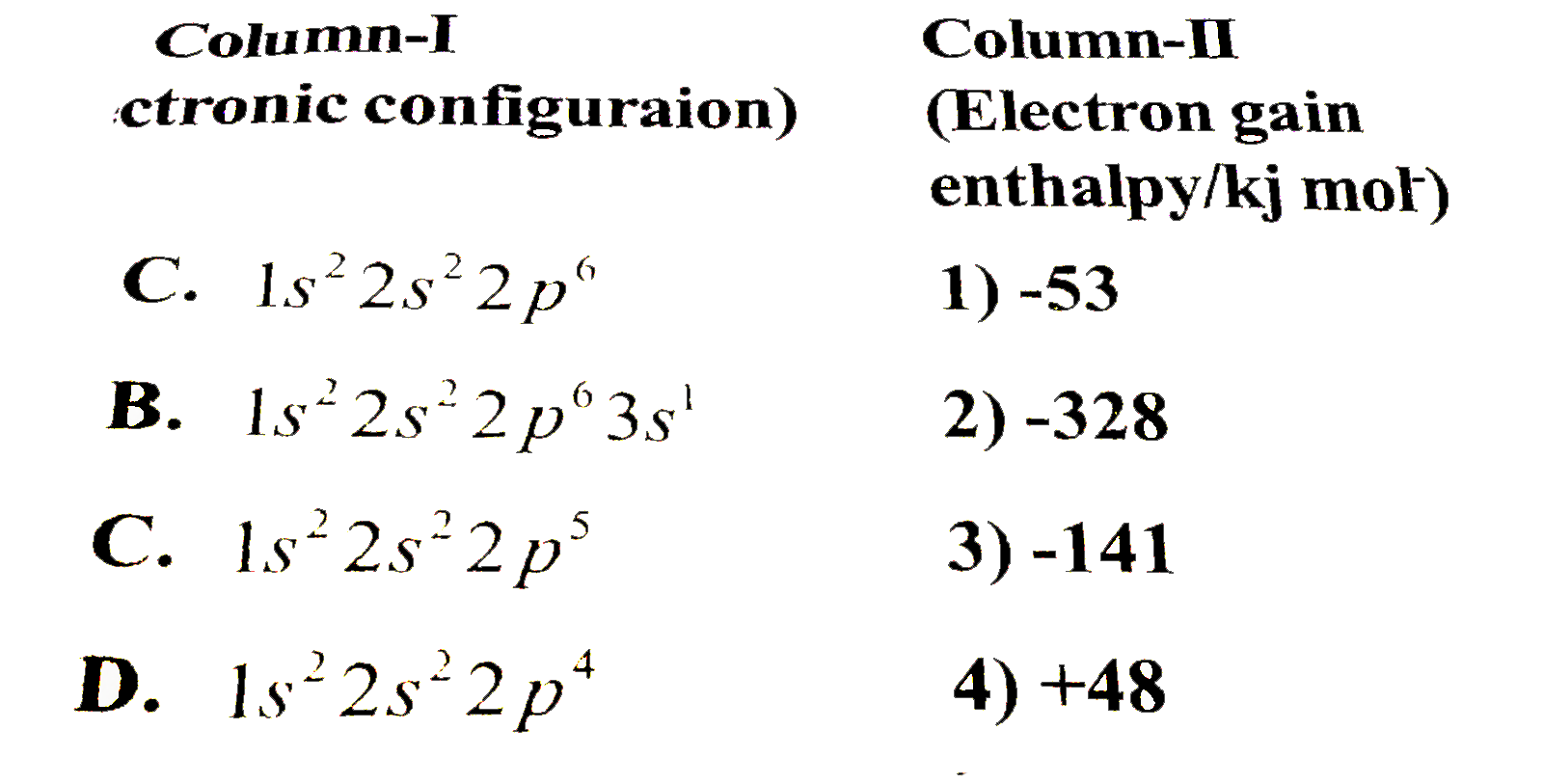A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 2 (H.W)|23 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 3|17 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 1 (H.W)|47 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 VideosENVIRONMENTAL CHEMISTRY
NARAYNA|Exercise EXCERCISE - IV (NCERT EXEMPLAR QUESTIONS)|7 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CLASSIFICATION OF ELEMENTS AND PERIODICITY -EXERCISE - 2 (C.W)
- Calculate the effective nuclear charge experienced by the 4s-electron ...
Text Solution
|
- Which of the following element is most electropositive?
Text Solution
|
- Which of the following elements has zero electron affinity ?
Text Solution
|
- The first ionization energy value of an element area 191, 578,872 and ...
Text Solution
|
- Which of the following elements represents highly electropositive as w...
Text Solution
|
- Although metals form basic oxides, which of the following metals form ...
Text Solution
|
- The order in which the following oxides are arranged according to decr...
Text Solution
|
- An element of atom mass 39 has the electron configuration 2,8,8,1 whic...
Text Solution
|
- Match the entries of Column I with appropriate entries of Column II an...
Text Solution
|
- Match the entries of Column I with appropriate entries of Column II an...
Text Solution
|
- Match the entries of Column I with appropriate entries of Column II an...
Text Solution
|
- Which of the following sequences contain atomic numbers of only repres...
Text Solution
|
- Which of the following elements will gain one electron more readily in...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Which of the following sets contain only isoelectronic ions?
Text Solution
|
- In which of the following options order of arrangement does not agree ...
Text Solution
|
- Which of the following have no unit?
Text Solution
|
- An element belongs to 3rd period and group 13 of the periodic table. W...
Text Solution
|
- Which is incorrectly matched
Text Solution
|
- Electronic configuration of some elements is given in Column I and the...
Text Solution
|