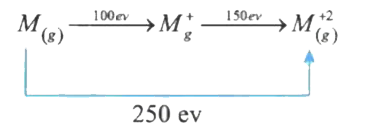A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 3|17 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 4|17 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 2 (C.W)|41 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 VideosENVIRONMENTAL CHEMISTRY
NARAYNA|Exercise EXCERCISE - IV (NCERT EXEMPLAR QUESTIONS)|7 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CLASSIFICATION OF ELEMENTS AND PERIODICITY -EXERCISE - 2 (H.W)
- The correct order of atomic radii is
Text Solution
|
- In which of the following arrangements, the order is not correct accor...
Text Solution
|
- Successive ionisation potentials of an element M are 8.3,25.1,37.9,259...
Text Solution
|
- The IP(1),IP(2),IP(3) and IP(4) of an element A are 6.0, 10.0, 16.0 an...
Text Solution
|
- H-H, X-X and H-X bond energies are 104 Kcal//"mole" 60 Kcal//"mole" a...
Text Solution
|
- The ionisation energy and electron affinity of an element are 13.0ev a...
Text Solution
|
- The bond energies of H-H, X-X and H-X are 104 K.cal, 38 K.cal and 138 ...
Text Solution
|
- The atomic numbers of elements A,B,C and D are Z - 1, Z, Z + 1 and Z +...
Text Solution
|
- M((g)) rarr M((g))^(+) +e^(-), DeltaH = 100eV M((g)) rarr M((g))^(2+...
Text Solution
|
- The increasing order of the first ionization enthalpies of the element...
Text Solution
|
- Using the data given below,predict the nature of heat changes for the ...
Text Solution
|
- The IE1 " and " IE2 of Mg (g) are 740 and 1450 kJ "mol:^(-1) . Calcul...
Text Solution
|
- How many Cs atoms can be convered to Cs^(+) ions by 1 joule energy if ...
Text Solution
|
- The elecron affinity of chlorine is 3. 7 eV. How much energy in kcal i...
Text Solution
|
- The energy needed for Li((g))to Li((g))^(+3)+3e^(-) is 1.96xx10^(4)"K...
Text Solution
|
- Following statements regarding the periodic trends of chemical reactiv...
Text Solution
|
- Which of the following represent the correct order of increasing first...
Text Solution
|
- The correct sequence which shows decreasing order of the ionic radii o...
Text Solution
|
- The set representing the correct order of ionic radius is
Text Solution
|
- The charge/size ratio of a cation determines its polarising power. Whi...
Text Solution
|