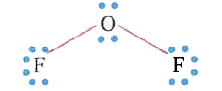
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- I (C.W.) (HYBRIDISATION)|14 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- I (C.W.) (DATIVE BOND)|6 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- I (C.W.) (VALENCE BOND THEORY)|6 VideosCARBONYL COMPOUNDS
NARAYNA|Exercise LEVEL -VI|99 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL BONDING AND MOLECULAR STRUCTURE-EXERCISE- I (C.W.) (VSEPR THEORY)
- CO2 is iso-structural with
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|
- In OF2, the number of bond pairs and lone pairs of electrons are respe...
Text Solution
|
- The geometry of ClO(3)^(-) ion according to valence shell electron pai...
Text Solution
|
- VSEPR Theory
Text Solution
|
- Which of the following molecule is linear?
Text Solution
|