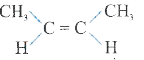A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- II (C.W.) (VALENCE BOND THEORY)|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- II (C.W.) (VSEPR THEORY)|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- II (C.W.) (RESONANCE)|3 VideosCARBONYL COMPOUNDS
NARAYNA|Exercise LEVEL -VI|99 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL BONDING AND MOLECULAR STRUCTURE-EXERCISE- II (C.W.) (BOND POLARITY AND DIPOLE MOMENT)
- The dipole moment of hydrogen chloride with bond distance 127 pm is 1....
Text Solution
|
- Which bond angle, theta would result in the maximum dipole moment for ...
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment ?
Text Solution
|
- Dipole moment is shown by
Text Solution
|
- Statement : The dipole moment of NH3 is less than NF3 . Explanation...
Text Solution
|
- The critical temperature of water is higher than that of O(2) because ...
Text Solution
|
- In which of the following bonds are polar but molecule is non-polar
Text Solution
|