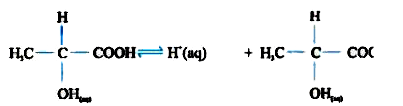A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -3|24 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -II (C.W.)|51 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE -4|54 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 4|17 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL EQUILIBRIUM-Exercise -II (H.W.)
- 1 mol of N2 and 4 mol of H2 are allowed to react in a vessel and after...
Text Solution
|
- At a certain temperature and a total pressure of 10^(5) Pa, iodine vap...
Text Solution
|
- The degree of ionization of 0.10 M lactic acid is 4.0% The value...
Text Solution
|
- The reaction of dimerisation of NO(2) in N(2)O(4) is 2NO(2)hArrN(2)O(4...
Text Solution
|
- A vessel at 1000 K contains carbon dioxide with a pressure of 0.5 atm....
Text Solution
|
- For an equilibrium reaction, N(2)O(4)(g) hArr 2NO(2)(g), the concentra...
Text Solution
|
- In the dissociation of PCl(5) as PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) ...
Text Solution
|
- Ag^(+) + NH(3) ltimplies [Ag(NH(3))]^(+), k(1)=6.8 xx 10^(-5) [Ag(NH...
Text Solution
|
- The most stable oxides of nitrogen will be :
Text Solution
|
- In the reaction PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g), the equilibrium c...
Text Solution
|
- The equilibrium constant for the given reaction: SO(3(g))hArrSO(2(g)...
Text Solution
|
- K(c) for 3//2H(2)+1//2N(2) hArr NH(3) are 0.0266 and 0.0129 atm^(-1), ...
Text Solution
|
- For the following reactions (1) , (2) and (3) equilibrium constants ar...
Text Solution
|
- For the reaction 2NO(2)(g) hArr 2NO(g)+O(2)(g) K(c)=1.8xx10^(-6) at ...
Text Solution
|
- The vapour pressure of water at 25°C is 0.0313 atm. Calculate the valu...
Text Solution
|
- One dm^(3) of hydrogen is present in a flask at a pressure of 10^(-12)...
Text Solution
|
- For the reaction at 25^(@)C, C(2)H4(g)+H(2)(g) LeftrightarrowC(2)H(6...
Text Solution
|
- In the dissociation of N(2)O(4) into NO(2), (1+ alpha) values with the...
Text Solution
|
- The degree of dissociation of PCl(5) will be more at ………. pressure.
Text Solution
|
- Before equilibrium is set-up for the chemical reaction, N(2)O(4)hArr 2...
Text Solution
|