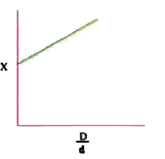
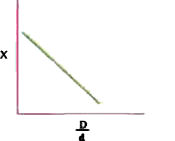
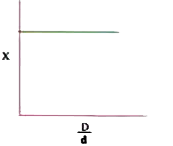
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -3|24 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -II (C.W.)|51 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE -4|54 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NARAYNA|Exercise EXERCISE - 4|17 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL EQUILIBRIUM-Exercise -II (H.W.)
- The equilibrium constant for the given reaction: SO(3(g))hArrSO(2(g)...
Text Solution
|
- K(c) for 3//2H(2)+1//2N(2) hArr NH(3) are 0.0266 and 0.0129 atm^(-1), ...
Text Solution
|
- For the following reactions (1) , (2) and (3) equilibrium constants ar...
Text Solution
|
- For the reaction 2NO(2)(g) hArr 2NO(g)+O(2)(g) K(c)=1.8xx10^(-6) at ...
Text Solution
|
- The vapour pressure of water at 25°C is 0.0313 atm. Calculate the valu...
Text Solution
|
- One dm^(3) of hydrogen is present in a flask at a pressure of 10^(-12)...
Text Solution
|
- For the reaction at 25^(@)C, C(2)H4(g)+H(2)(g) LeftrightarrowC(2)H(6...
Text Solution
|
- In the dissociation of N(2)O(4) into NO(2), (1+ alpha) values with the...
Text Solution
|
- The degree of dissociation of PCl(5) will be more at ………. pressure.
Text Solution
|
- Before equilibrium is set-up for the chemical reaction, N(2)O(4)hArr 2...
Text Solution
|
- For reacrtion 2NOC1(g) hArr 2NO(g) + C1(2)(g) , K(c) at 427^(@)C is 3x...
Text Solution
|
- N(2)O(4) is dissociated to 33% and 50% at total pressure P(1) and P(2)...
Text Solution
|
- The following equilibrium constants are given : N(2) + 3 H(3) hArr ...
Text Solution
|
- For the reaction, PCl(3) (g)+Cl(2) (g) Leftrightarrow PCl(5) (g)" at "...
Text Solution
|
- The equilibrium constant K(p(1)) and K(p(2)) for the reactions XhArr2Y...
Text Solution
|
- Rate of diffucion of ozonized oxygen is 0.4sqrt5 times that of pure ox...
Text Solution
|
- For the reaction, SO(2)(g) + (1)/(2)O(2)(g)hArrSO(3) (g), If K(p) = K(...
Text Solution
|
- For the reaction, N(2)O(4)(g)hArr 2NO(2)(g) the reaction connectin...
Text Solution
|
- The prepation of SO(3)(g) by reaction SO(2)(g)+(1)/(2)O(2)(g)hArrSO(3)...
Text Solution
|
- For a reaction at equilibrium PCl(5(g)) Leftrightarrow pcL(3(G))+Cl(2(...
Text Solution
|