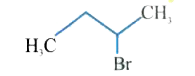
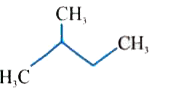
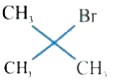
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NARAYNA|Exercise EVALUATE YOURSELF - 2|2 VideosHYDROCARBONS
NARAYNA|Exercise EVALUATE YOURSELF - 3|2 VideosHYDROCARBONS
NARAYNA|Exercise EXAMPLE|8 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Matrix-Match type questions|7 VideosHYDROGEN & ITS COMPOUCDS
NARAYNA|Exercise Interger type questions|10 Videos
Similar Questions
Explore conceptually related problems