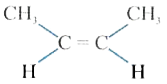
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NARAYNA|Exercise EXERCISE - 2 (H.W) (PREPARATION & PROPERTIES)|5 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 3|25 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 2 (H.W) (ALKENES) (PREPARATIONS OF ALKENES)|2 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Matrix-Match type questions|7 VideosHYDROGEN & ITS COMPOUCDS
NARAYNA|Exercise Interger type questions|10 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HYDROCARBONS -EXERCISE - 2 (H.W) (PROPERTIES OF ALKENES)
- In the following sequence of reactions the products D is C-=CH overs...
Text Solution
|
- 1-Butyne on reductive ozonolysis gives.
Text Solution
|
- Which of the following compound has the lowest dipole moment
Text Solution
|
- CH-=CHoverset(NaNH(2))to[A]overset(CH(3)Br)to[B]
Text Solution
|
- Hydration of ethyne to ethanal takes place through the formation of
Text Solution
|
- A compound on dehydrohalogenatio with alcoholic KOH gives alkyne but o...
Text Solution
|
- CaC(2)overset("Hydrolysis")toAoverset(HgSO(4)+dil.H(2)SO(4))toB. B is
Text Solution
|
- CaC(2)+H(2)OtoA+Boverset("1 mole "Na)toCoverset(C(2)H(5)I)toD. D is
Text Solution
|
- underset(Cl)underset(|)(C)H(2)-underset(Cl)underset(|)(C)H(2)overset(A...
Text Solution
|
- CH-=Choverset(HCl)toAoverset("Polymerisation")toB The polymer B is
Text Solution
|
- H-C-=C-H+ 2NaNH(2)rarr Aoverset("2 mole")underset(CH(3)Cl)rarrB then...
Text Solution
|
- When 2-pentyn is treated with dilute H(2)SO(4) and HgSO(4) the product...
Text Solution
|
- The cyclic polymerisation of methyl acetylene produces
Text Solution
|
- The compounds 1-butyne and 2-butyne can be distinguished by using
Text Solution
|
- Which of the following orders regarding acidic strength is correct
Text Solution
|
- Anunknown compound 'A' has a molecular formula of C(4)H(6) when 'A' is...
Text Solution
|
- The reduction of 4 - octyne with H(2) in the presence of Pd //CaCo3 qu...
Text Solution
|
- The hydrolysis of Mg(2)C(3) produces
Text Solution
|
- Pure acetylene has sweet smell, where as impure gives garlic occur du...
Text Solution
|
- The stronger base is
Text Solution
|