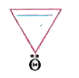
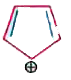
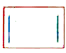A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NARAYNA|Exercise EXERCISE - 2 (H.W) (PREPARATION & PROPERTIES)|5 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 3|25 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 2 (H.W) (ALKENES) (PREPARATIONS OF ALKENES)|2 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Matrix-Match type questions|7 VideosHYDROGEN & ITS COMPOUCDS
NARAYNA|Exercise Interger type questions|10 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HYDROCARBONS -EXERCISE - 2 (H.W) (PROPERTIES OF ALKENES)
- 1-butyne on reaction with hot alkaline KMnO(4) gives:
Text Solution
|
- Order of acidity of H(2)O, NH(3) and acetylene is :
Text Solution
|
- Which of the following is expected to aromatic
Text Solution
|
- Which of the following carbocation is expected to be least stable
Text Solution
|
- Which of the following carbocations is expected to be most stable ?
Text Solution
|
- Which of the following structure will not have 4pi electrons
Text Solution
|
- CaC(2)overset(H(2)O)toAoverset("Red tube hot")toBunderset(CH(3)Cl)over...
Text Solution
|
- C(2)H(2)overset("Red hot tube")toAoverset("fuming "H(2)SO(4))toB then ...
Text Solution
|
- Aoverset("soda lime")toC(6)H(6)overset(Cl(2),hv)toB, In this reaction ...
Text Solution
|
- A & B respectively are
Text Solution
|
- The descending order of reactivity of C(2)H(6), C(2)H(4), C(2)H(2) and...
Text Solution
|
- A new carbon-carbon bond is formed in
Text Solution
|
- X overset("Dil. "H(2)SO(4))underset("Boil")rarr Yoverset("Zndust")unde...
Text Solution
|
- In which of the following reactions, aromatic character is retained?
Text Solution
|
- Number of sigmasp^(2)-sp^(2) bonds present in a molecule of X in the p...
Text Solution
|
- What is 'X' in the following reaction ? C(6)H(5)-C-=C-H overset(Hg^(...
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory ,
Text Solution
|
- The electrophile in Acetylation of Benzene is
Text Solution
|
- Four structures are given in option (1) to (4). Examine them and selec...
Text Solution
|
- Which of the following is the correct IUPAC Name of the compound
Text Solution
|