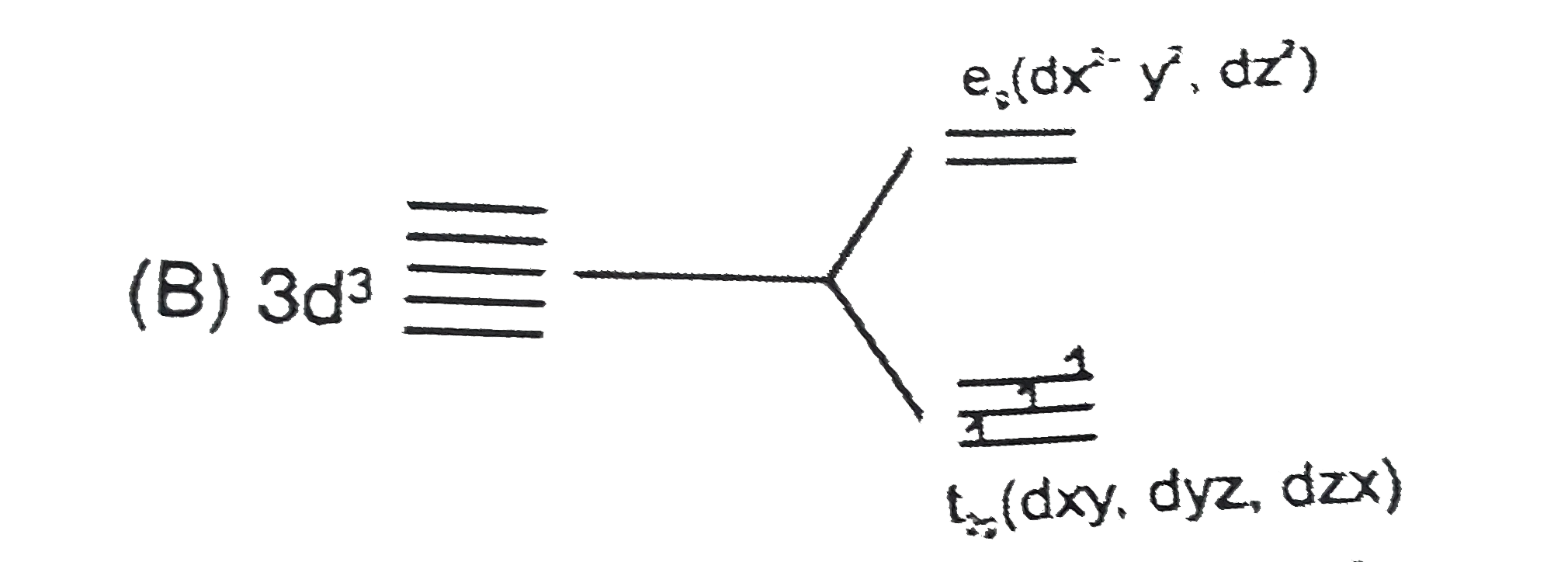
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-3) (One Integer Value Correct Type )|5 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-4) (Paragraph for Question )|3 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-1)|7 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-2)
- Which of the following statement(s) is/are correct ?
Text Solution
|
- Which of the following is true for the complex Co(NO(2))(Cl)(2).5NH(3)...
Text Solution
|
- Which of the following complexes can exists as diastereoisomer ?
Text Solution
|
- Tetrahedral complexes are generally favoured:
Text Solution
|
- Which of the following statement is/are incorrect for the complex [Cr(...
Text Solution
|