Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Conceptual Questions (2)|21 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Conceptual Questions (3)|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Practice Problems|29 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Conceptual Questions (1)
- Out of MgO and NaCl, which has higher lattice energy and why?
Text Solution
|
- Why is NaCl a bad conductor of electricity in the solid state?
Text Solution
|
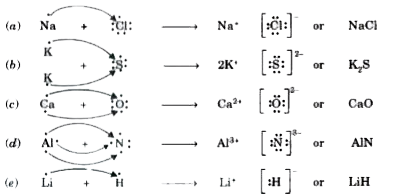
- Use Lewis dot symbols to show electron transfer between the following ...
Text Solution
|
- Write Lewis dot symbols for the following elements : Mg, Na, B, O, N...
Text Solution
|
- Write Lewis symbol for the following atoms and ions : S and S^(2-),"...
Text Solution
|
- Write Leiws dot symbols for O and O^(2-)
Text Solution
|
- Which of the two is more hard :MgO or CaO? The internuclear distances ...
Text Solution
|
- Why does NaCl give a white precipitate with AgNO(3) solution but C Cl(...
Text Solution
|
- Calculate the formal charge on each atom in :overset(..)underset(..)...
Text Solution
|
- Identify the compound/compounds in which S does not obey octet rule : ...
Text Solution
|
- How is lattice enthalpy related to stability of an ionic compound?
Text Solution
|
- Give one example of a molecule not obeying octet rule.
Text Solution
|