A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
MODERN PUBLICATION|Exercise Objective C.(MCQs)|11 VideosEQUILIBRIUM
MODERN PUBLICATION|Exercise Objective D.(MCQs) Passage|10 VideosEQUILIBRIUM
MODERN PUBLICATION|Exercise Objective A.(MCQs)|41 VideosENVIRONMENTAL POLLUTION
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|15 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|12 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-EQUILIBRIUM-Objective B.(MCQs)
- Which of the following salts is the most basic in aqueous solution?
Text Solution
|
- An alkali is titrated against an acid with methyl orange as indicator,...
Text Solution
|
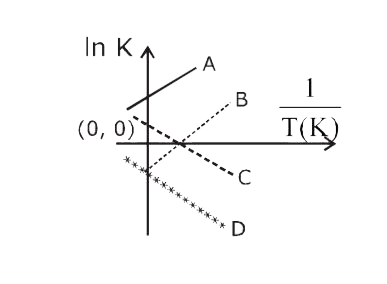
- Which of the following lines correctly show the temperature dependence...
Text Solution
|
- In a chemical reaction , A+2Boverset(K)hArr2c+D, the initial concent...
Text Solution
|
- If K(sp) of Ag(2)CO3 is 8xx10^(-12), the molar solubility of Ag2CO3 in...
Text Solution
|
- CH3COOH is 1% ionised in its aqueous solution of 0.1 M strength . Its ...
Text Solution
|
- The equilibrium constant of the reaction, H(2)+I(2)hArr 2HI is 50. If ...
Text Solution
|
- The pH of pure water at 90^@C will be
Text Solution
|
- The equilibrium constant for the reaction, N(2(g))+3H(2(g)) to 2NH(3(g...
Text Solution
|
- At a certain temperature 2 moles of carbonmonoxide and 3 moles of chlo...
Text Solution
|
- The solubility of AgCl will be miniumum in
Text Solution
|
- The values of K(p)//K(c) for the following reactions at 300 K are, res...
Text Solution
|
- 20 mL of 0.1 M H2SO4 solution is added to 30 mL of 0.2 M NH4OH solutio...
Text Solution
|
- 5.1 g NH(4)SH is introduced in 3.0 L evacuated flask at 327^(@)C, 30%...
Text Solution
|
- For the equilibrium, 2H(2)O hArr H(3)O^(+) + OH^(-),the value of De...
Text Solution
|
- For the following reactions , equilibrium constants are given : S(s)...
Text Solution
|
- The molar solubility of Cd(OH)(2) is 1.84xx10^(-5)M in water. The exp...
Text Solution
|
- Consider the following reversible chemical reactions : A(2)(g)+B(2)...
Text Solution
|
- Consider the following statements (A)The pH of a mixture containing ...
Text Solution
|
- Two solids dissociates as follows A(s) rarr B(g) + C(g), K(P(1)) = x a...
Text Solution
|