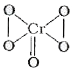
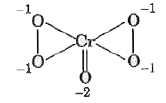
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - III )|2 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise Recent Examination Questions|6 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise Recent Examination Questions|6 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
MODERN PUBLICATION|Exercise Recent Examination Question|4 VideosSOLUTIONS
MODERN PUBLICATION|Exercise Recent Examination Questions|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-REDOX REACTIONS-Multiple Choice Questions (Level - II )
- The oxidation states of sulphur in the anions SO(3)^(-2),S(2)O(4)^(2-)...
Text Solution
|
- Oxidation number of iodine in IO(3),IO(4),KI andI(2) respectively are
Text Solution
|
- Oxidation state of chromium in
Text Solution
|
- Using the standard electrode potential , find out the pair between whi...
Text Solution
|
- The standard electrode potentials of some electrodes are : {:("Electro...
Text Solution
|
- For the redox reaction : MnO(4)^(-)+C(2)O(4)^(2-)+H^(+)toMn^(2+)+CO(...
Text Solution
|
- The oxidation number of S in S(8) , S(2)F(2)andH(2)S respectively are ...
Text Solution
|
- Amongst the following identify the species with an atom with +6 oxidat...
Text Solution
|
- In the electrolytic cell , flow of electrons is from
Text Solution
|
- In the balance chemical equation : IO(3)^(-)aI^(-)+bH^(+)tocH(2)O+dI...
Text Solution
|
- The standard oxidation potentials of Zn, Cu, Ag and Ni electrodes are ...
Text Solution
|
- The elctrochemical cell stops working after sometime because
Text Solution
|
- On the basis of following E^(@) values , the strongest oxidising agent...
Text Solution
|
- The oxidation number of sulphur atoms in peroxomonosulphuric acid (H(2...
Text Solution
|
- Oxidation number of P in PO(4)^(3-),S" in "SO(4)^(2-) and that of Cr ...
Text Solution
|
- In which of the following compounds , nitrogen exhibits highest oxidat...
Text Solution
|
- A compound contains atoms of three elements A,B and C. If the oxidatio...
Text Solution
|
- Identify disproportionation reaction :
Text Solution
|
- When KMnO(4) acts as an oxidising agent and ultimately forms MnO(4)^(...
Text Solution
|
- Which of the following is a redox reaction ?
Text Solution
|