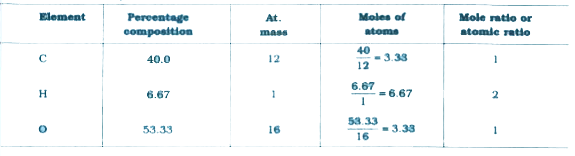
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS-Recent Examination Question
- In Kjeldahl's method, ammonia from 5g of food neutralizes 30cm^(3) of ...
Text Solution
|
- 1.2g of an organic compound on Kjeldahlization liberates ammonia which...
Text Solution
|
- 0.30g of an organic compound containing C, H and Oxygen on combustion...
Text Solution
|
- An organic comound weighing 0.15g on Carius estimation gave 0.12g of ...
Text Solution
|