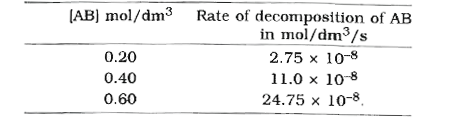A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level-III)|9 VideosCHEMICAL KINETICS
MODERN PUBLICATION|Exercise Recent Examination Questions|20 VideosCHEMICAL KINETICS
MODERN PUBLICATION|Exercise Multiple Choice Questions ( Effect of Temperature Catalyst and Radiation on Rate of Reaction )|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Recent Examination Questions|17 VideosCHEMISTRY IN EVERY DAY LIFE
MODERN PUBLICATION|Exercise Recent Examination Questions|4 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL KINETICS -Multiple Choice Questions (Level-II)
- The time taken for 10% completion of a first order reaction is 20 min ...
Text Solution
|
- For a chemical raction AtoB the rate of the reaction is 2xx10^(-3) mol...
Text Solution
|
- For the decomposition of a compound AB at 600 K the following data wer...
Text Solution
|
- For a fist order reaction the rate constant is 6.909 min^(-1) . The ti...
Text Solution
|
- The activation energies of two reactions are E(1) and E(2) (E(1) gt E(...
Text Solution
|
- In the reaction BrO(3)^(-)(aq)+ 5Br^(-)+6H^(+) rarr 3Br(2)+3H(2)O ...
Text Solution
|
- Consider the chemical reaction : N(2)(g) +3H(2)(g) rarr 2NH(3)(g) ...
Text Solution
|
- In a first order reaction the concentration of the reactant decreases ...
Text Solution
|
- The reaction : Xrarr Product follows first order kinetics . In 40...
Text Solution
|
- In a first order reaction the concentration of the reactant decreases ...
Text Solution
|
- A schematic plot of In k(eq) versus inverse of temperature for a react...
Text Solution
|
- Consider an endothermic reaction : XrarrY with activation energie...
Text Solution
|
- Consider a reaction a G + bH rarr Products . When concentration of bot...
Text Solution
|
- The energies of activation for forward and reverse reactions for A(2)+...
Text Solution
|
- For reaction Ararr B the rate of reaction incrases by a factor of 1.8...
Text Solution
|
- For a reaction NO(g) +O(2) (g) rarr 2NO(2)(g) Rate =k[NO^(2)] [O(2...
Text Solution
|
- For a first order reaction involving decomposition of N(2) O(5) the fo...
Text Solution
|
- Which of the following graphs correspond to first order reaction ?
Text Solution
|
- For a second order reaction rate at a particular time is x . If the in...
Text Solution
|
- For a reaction having rate law expression : Rate = k[A]^(3//2) [B]^(...
Text Solution
|