Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
MODERN PUBLICATION|Exercise Practice problems|17 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise Conceptual Questions|20 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS ( based on the given. passage/comprehension )|8 VideosS-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS )
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
MODERN PUBLICATION-REDOX REACTIONS -COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)
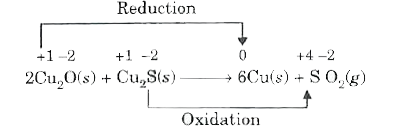
- Justify that the reaction 2Cu(2)O(s)+Cu(2)S(s)rarr6Cu(s)+SO(2)(g) a ...
Text Solution
|
- Which of the following are redox reaction ?
Text Solution
|
- In which of the following the oxidation number of atom is/are correctl...
Text Solution
|
- Which of the following act both as an oxidising as well as reducing ag...
Text Solution
|
- In which of the following the oxidation number of the underlined atom ...
Text Solution
|
- Oxidation number of Cr in CrO5 is same as of S in
Text Solution
|
- Which of the following statements are not correct for the following re...
Text Solution
|
- Which of the following statements are wrong ?
Text Solution
|
- Consider the redox reaction 2S(2)O(3)^(2-)+I(2)rarrS(4)O(6)^(2-)+2I^...
Text Solution
|
- For the reaction : I^(-) +ClO3^(-)+H2SO4rarr Cl^(-)+HSO4^(-)+I2 Th...
Text Solution
|