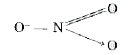Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
MODERN PUBLICATION|Exercise NCERT FILE Solved (NCERT - Exemplar Problems)) (Multiple Choice Questions (Type - I)|1 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise NCERT FILE Solved (NCERT - Exemplar Problems) (Multiple Choice Questions (Type - I)|10 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise Conceptual Questions|20 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS ( based on the given. passage/comprehension )|8 VideosS-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS )
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-REDOX REACTIONS -NCERT FILE Solved (Textbook Exercises)
- Justify that the following reaction are redox reactions: a. CuO(s)+H...
Text Solution
|
- Fluorine reacts with ice and results in the change: H(2)O(s)+F(2)(g)...
Text Solution
|
- Calculate the oxidation number of sulphur, chromium, and nitrogen in H...
Text Solution
|
- Write formulas for the following compounds (a) Mercury (II) chloride...
Text Solution
|
- Consider the reactions : (a) 6CO2(g) +6H2O(l) rarr C6H(12)O6(aq) +6O...
Text Solution
|
- The compound AgF2 is unstable compound . However , if formed , the com...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- How do you count for the following observations ? (a) Though alkali...
Text Solution
|
- Identify the substance oxidised, reduced , oxidising agent and reducin...
Text Solution
|
- Consider the reaction: 2S(2)O(3)^(2-)(aq)+I(2)(s) rarr S(4)O(6)^(2-)...
Text Solution
|
- Justify giving reaction that among halogens, fluorine is the best oxid...
Text Solution
|
- Why does the following reaction occur? XeO(6)^(4-)(aq)+2F^(Θ)(aq)+6H...
Text Solution
|
- Consider the reactions: a. H(3)PO(2)(aq)+4AgNO(3)(aq)+2H(2)O(l) rarr...
Text Solution
|
- Balance the following redox reactions by ion electron method: a. MnO...
Text Solution
|
- Balance the following equations in basic medium by ion-electron method...
Text Solution
|
- What sort of informations can you draw from the following reaction? ...
Text Solution
|
- The Mn^(3+) ion is unstable in solution and undergoes disproportionati...
Text Solution
|
- Consider the elements : Ca, Na, I and F Identify the elements tha...
Text Solution
|
- Chlorine is used purify drinking water . Excess of chlorine is harmful...
Text Solution
|
- Refer to the periodic table given in your book and now answer the foll...
Text Solution
|