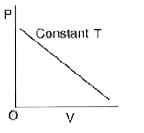
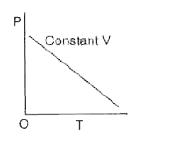
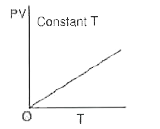
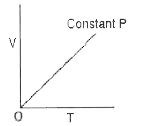
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE (MAIN) & OTHER STATE BOARDS FOR ENGINEERING ENTRANCE)|44 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE (ADVANCED) FOR IIT ENTRANCE)|8 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (A. MULTIPLE CHOICE QUESTIONS)|41 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 VideosSTRUCTURE OF ATOM
MODERN PUBLICATION|Exercise Unit Practice Test|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-COMPETITION FILE OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)
- If the ration of the masses of SO(3) and O(2) gases confined in a vess...
Text Solution
|
- Which of the following diagrams correctly decribes the behavior of a f...
Text Solution
|
- A bottle of dry ammonia and a bottle of dry hydrogen chloride connecte...
Text Solution
|
- A 4.0 dm^(3) flask containing N(2) at 4 bar was connected to a 6.0 dm...
Text Solution
|
- If a gas expands at constant temperature, it indicates that
Text Solution
|
- Critical temperature of H(2)O, NH(3), CO(2) and O(2) are 647 K, 405.6 ...
Text Solution
|
- What will happen to volume of a bubble of air found under water in a l...
Text Solution
|
- The incorrect statement among the following
Text Solution
|
- An evacuated vessel weighs 50 g when empty, 144 g when filled with a l...
Text Solution
|
- The mass of 2.24xx10^(-3)m^(3) of a gas is 4.4 g at 273.15 K and 101.3...
Text Solution
|
- A bubble of gas released at the bottom of a lake increases to eight ti...
Text Solution
|
- A gaseous mixture was prepared by taking equal moles of CO and N(2). I...
Text Solution
|
- Two gases A and B having the same volume diffuse through a porous part...
Text Solution
|
- By what factor does the average velocity of a gaseous molecule increas...
Text Solution
|
- A mixture contains 64 g of dioxygen and 60 g of neon at a total pressu...
Text Solution
|
- Choose the incorrect statement in the following
Text Solution
|
- The gas with the highest critical temperature is
Text Solution
|
- 50 mL of each gas A and of gas B takes 150 and 200 seconds respectivel...
Text Solution
|
- The compressibility factor for a real gas at high pressure is .
Text Solution
|
- The average energy per molecule of a gas at a given temperature, T, is...
Text Solution
|