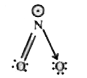
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 1: Electrovalent, Covalent and Coordinate Bonding)|17 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 2: Octet Rule, Resonance, Dipole Moment and Bond Polarity)|13 VideosCHAPTERWISE NUMERIC/INTEGER ANSWER QUESTIONS
DISHA PUBLICATION|Exercise CHAPTER 28- BIOMOLECULES|10 VideosCHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 2 : CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -EXERCISE-2: CONCEPT APPLICATOR
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- Correct set of species with zero dipole moment is : (i) CO(2) " "...
Text Solution
|
- Match List I and List II and pick out correct matching codes from the ...
Text Solution
|
- Which of the following is correct order of sigma - bond strength ? ...
Text Solution
|
- In pyrophosphoric acid, H(4)P(2)O(7), number of sigma and dpi -p pi
Text Solution
|
- Arrange the following ions in the order of decreasing X-O bond length ...
Text Solution
|
- Which of the following statements is correct in the context of the all...
Text Solution
|
- Which of the following set contains species having same angle around t...
Text Solution
|
- Bond angle between two hybrid orbitals is 105^@ Percentage of s-orbita...
Text Solution
|
- The group of molecules having identical shape is:
Text Solution
|
- The shapes of XeF(4),XeF(5)^(-) and SnCl(2) are :
Text Solution
|
- Which of these statements is not true?
Text Solution
|
- The statement true for N(3)^(-) is
Text Solution
|
- The dipole moments of diatomic molecules AB and CD are 10.41D and 10.2...
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|
- The charge/size ratio of a cation determines its polarizing power. Whi...
Text Solution
|
- The resultant dipole moment (mu) of two compounds NOF and NO(2)F is 1....
Text Solution
|
- The BCl(3) is a planar molecule whereas NCI(3) is pyramidal because
Text Solution
|
- The cylindrical shape of alkynes is due to
Text Solution
|
- The AsF5 molecule is trigonal bipyramidal. The orbitals used by As for...
Text Solution
|
- The correct order of O - O bond length in O(2)H(2)O(2) and O(3) is
Text Solution
|