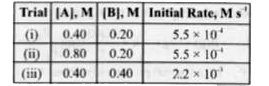
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 1 : CONCEPT BUILDER (TOPICWISE) (TOPIC 2: Order of Reaction and Half Life Period)|32 VideosCHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 1 : CONCEPT BUILDER (TOPICWISE)(TOPIC 3 : Theories of Rate of Reaction)|17 VideosCHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 2 : CONCEPT APPLICATOR|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-2: CONCEPT APPLICATOR|30 VideosCHEMISTRY IN EVERDAY LIFE
DISHA PUBLICATION|Exercise Exercise|88 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-CHEMICAL KINETICS -EXERCISE 1 : CONCEPT BUILDER (TOPICWISE) (TOPIC 1: Rate of Reaction, Rate Laws and Rate Constant)
- Which of the following will react at the highest rate ?
Text Solution
|
- Burning of coal is represented as C(s)+O(2)(g)rarr CO(2)(g). The rate ...
Text Solution
|
- If n(A) and n(B) are the number of moles at any instant in the reactio...
Text Solution
|
- Rate of a reaction
Text Solution
|
- If 3A rarr 2B, then the rate of reaction of + (dB)/(d t) is equal to
Text Solution
|
- for the reaction, 2A + B rarr 3C + D, which of the following does not ...
Text Solution
|
- For the reaction, N2O5(g) rarr 2NO2(g)+1//2O2(g), the value of rate of...
Text Solution
|
- The rate of a reaction increases four-fold when the concentration of r...
Text Solution
|
- k(34^(@))/k(35^(@)) lt 1, then
Text Solution
|
- The rate of the reaction 2NO+CI(2)rarr 2NOCI is given by the rate ...
Text Solution
|
- For a reaction A+B to C +2D, experimental results were collected for ...
Text Solution
|