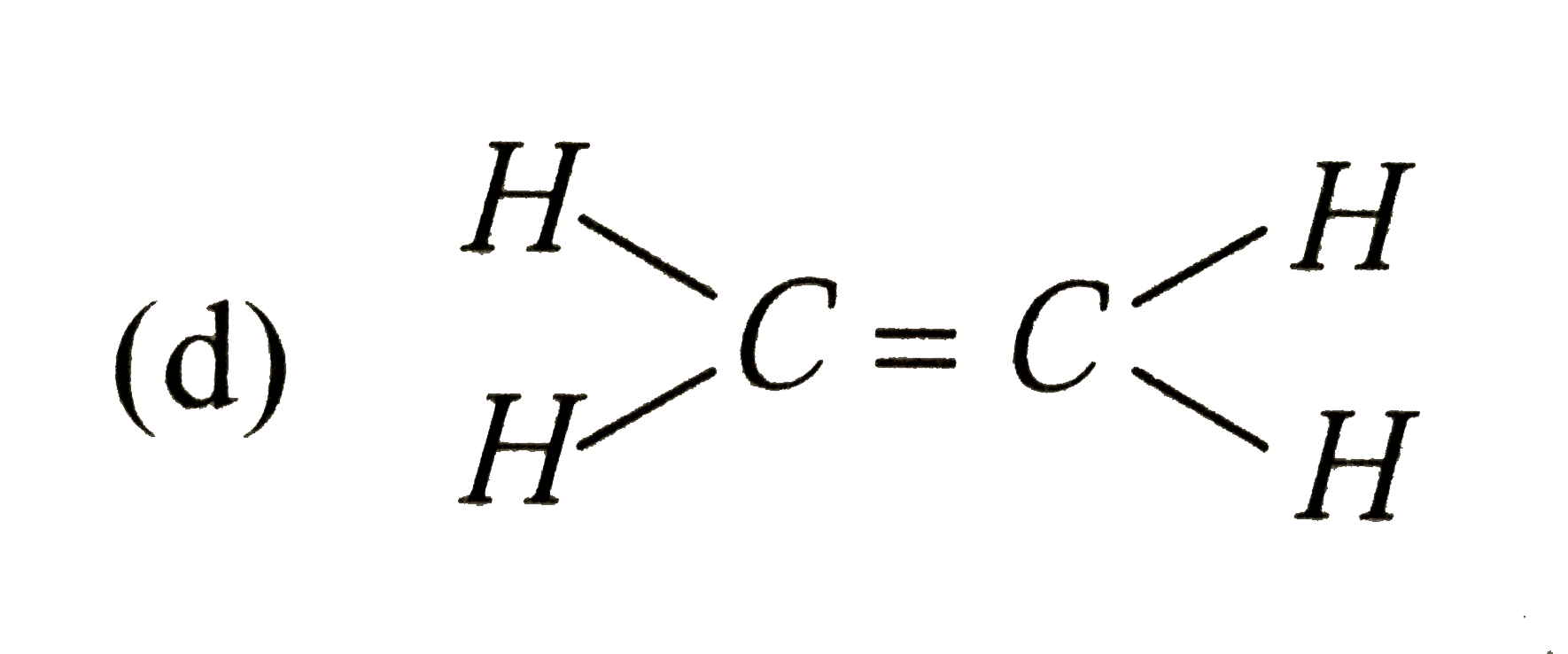A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS |Exercise Organic reactions and their mechanism|67 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS |Exercise Structural and stereo isomerism|140 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS |Exercise Matrix Match type questions|4 VideosGASEOUS STATE
ERRORLESS |Exercise JEE SECTION JEE (Advanced) 2018 Numeric answer type question|1 VideosHALOGEN CONTAINING COMPOUND
ERRORLESS |Exercise Critical Thinking (Jee (Advanced) 2018)|1 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -GENERAL ORGANIC CHEMISTRY-Dipole moment, resonance and reaction intermediates
- Hyperconjugation is also known as
Text Solution
|
- Relative stabilities of the following carbocations will be in the orde...
Text Solution
|
- Which of the following molecules does not have net dipole moment?
Text Solution
|
- Compound which shows positive mesomeric effect
Text Solution
|
- Aromatic properties of benzene are proved by
Text Solution
|
- Amongst the given structures , which are permissible resonance forms ?
Text Solution
|
- Which one of the following will be the most easiliy attacked by an ele...
Text Solution
|
- Which compound shows dipole moment
Text Solution
|
- The species responsible for nitration is
Text Solution
|
- C-C' bond length in benzene lies between single and double bond. The r...
Text Solution
|
- Choose the chain terminating step (1) H(2) rarr H^(*)+H^(*) (2) Br...
Text Solution
|
- Arrangements of (CH(3))(3)C-,(CH(3))(2)CH-,CH(3).CH(2)- when attached ...
Text Solution
|
- Due to the presence of an unpaired electron, free radicals are:
Text Solution
|
- The decreasing order of nucleophilicity among the nucleophiles is : ...
Text Solution
|
- The increasing order of stability of the following free radicals is :
Text Solution
|
- Among the following mixiture dipole-dipole as the mojor interaction is...
Text Solution
|
- Arrange the carbonions, (CH(3))(3)overset(-)C, overset(-)C Cl(3), (C...
Text Solution
|
- In carbonium ion the carbon bearing the positive charge in the
Text Solution
|
- Which of the following is observed in ethylene molecule
Text Solution
|
- Which of the following is a polar compound
Text Solution
|