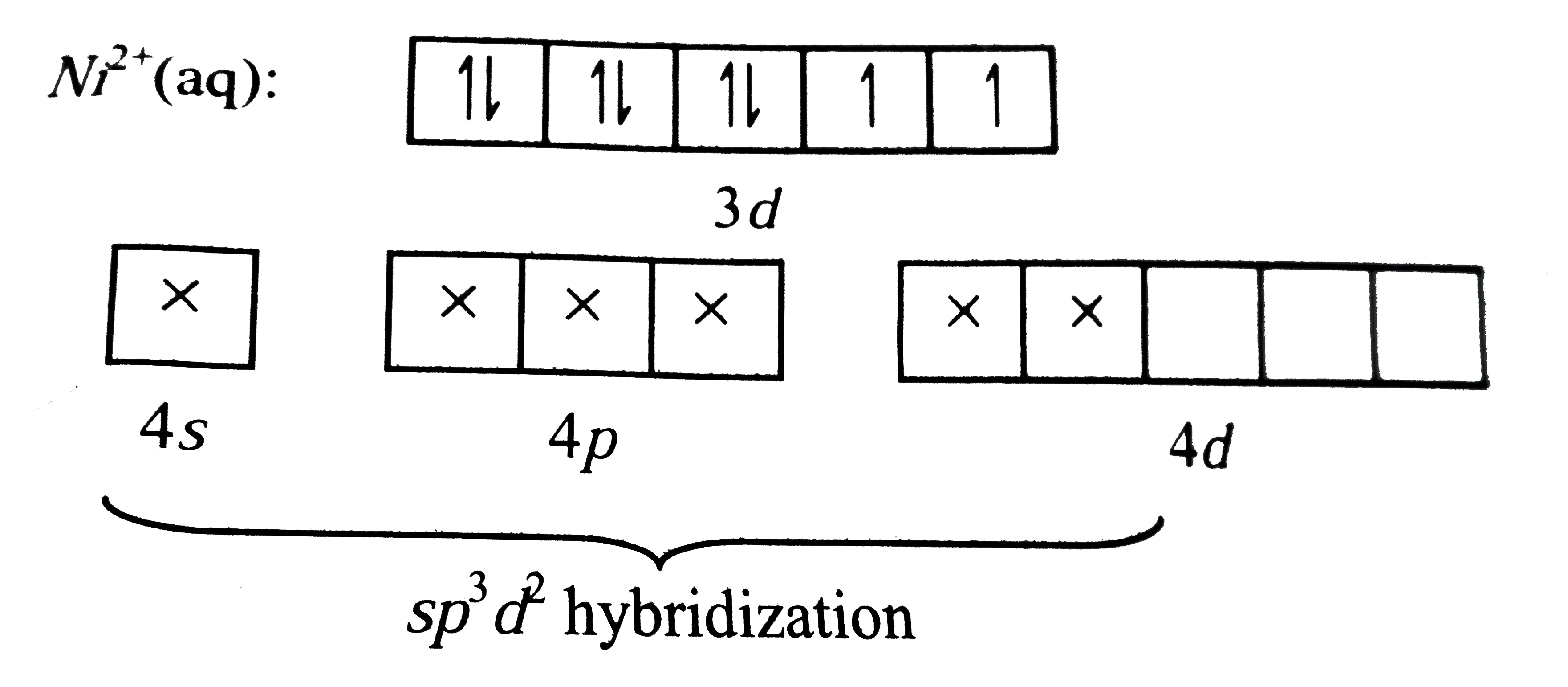
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION CHEMISTRY
ERRORLESS |Exercise ORDINARY THINKING Objective Questions (HYBRIDISATION AND GEOMETRY)|53 VideosCOORDINATION CHEMISTRY
ERRORLESS |Exercise ORDINARY THINKING Objective Questions (COMPLEXES AND COMPLEX STABILITY)|17 VideosCOORDINATION CHEMISTRY
ERRORLESS |Exercise ORDINARY THINKING Objective Questions (Nomenclature, Oxidation state and EAN number)|58 VideosCLASSIFICATION OF ELEMENTS AND PERIODIC PROPERTIES
ERRORLESS |Exercise JEE Section Matrix Match type questions|2 VideosD & P-BLOCK ELEMENTS
ERRORLESS |Exercise JEE Section (Numeric answer type questions)|2 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -COORDINATION CHEMISTRY-ORDINARY THINKING Objective Questions (Isomerism and Magnetic properties)
- Magnetic moment of [Cu(NH(3))(4)]^(2+) ion is
Text Solution
|
- The type of isomerism present in nitropentaamminechromium(III) chlorid...
Text Solution
|
- The "spin-only" magnetic moment [in units of Bohr magneton, (muB)] or...
Text Solution
|
- The magnetic moment (spin only) of [NiCl(4)]^(2-) is
Text Solution
|
- Which of the following has an optical isomer?
Text Solution
|
- Which of the following pairs represents linkage isomers
Text Solution
|
- The magnetic moment of salt containing Zn^(2+) ion is :
Text Solution
|
- The ion of least magnetic moment among the following is
Text Solution
|
- The optically active co-ordination complex ion among the following is
Text Solution
|
- The complesx [CoF(6)]^(4-) is
Text Solution
|
- Which is high spin complex
Text Solution
|
- For the given complex [CoCl(2)(en)(NH(3))(2)]^(+), the number of geome...
Text Solution
|
- Which of the following can participate in linkage isomerism
Text Solution
|
- The magnetic moment of a transition metal is sqrt(15) B.M . Find out ...
Text Solution
|
- The spin only magnetic moment of Mn^(4+) ion is nearly
Text Solution
|
- Amongst [NiCl(4)]^(2-), [Ni(H(2)O)(6)]^(2+), [Ni(PPh(3))(2)Cl(2)], [Ni...
Text Solution
|
- The spin-only magnetic moment of [CrF(6)]^(4) ( atomic number of Cr is...
Text Solution
|
- What is the magnetic moment of K(3)[FeF(6)] ?
Text Solution
|
- How many unpaired electrons are present in the central metal ion of [C...
Text Solution
|
- The reaction [Fe(CN)(6)]^(3-) rarr [FeF(6)]^(3-) takes place with
Text Solution
|