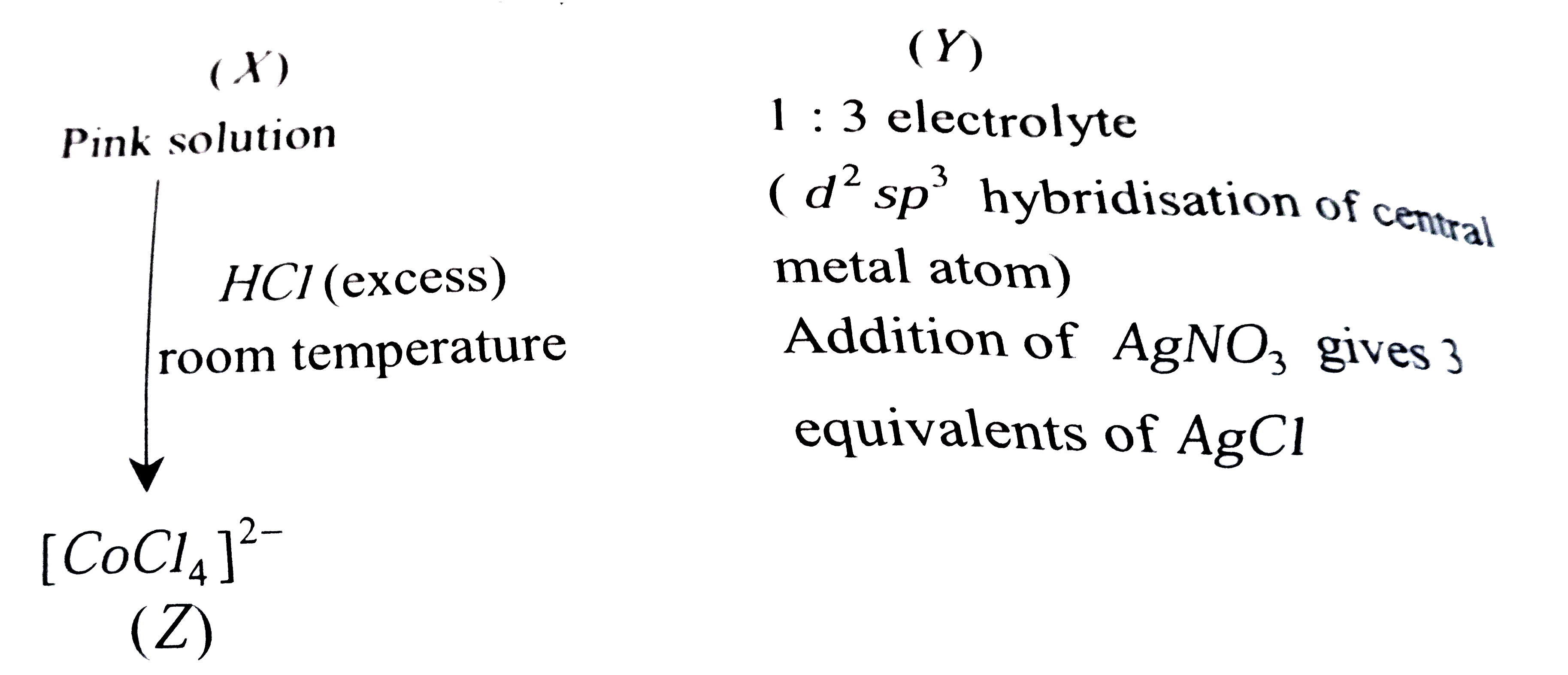A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION CHEMISTRY
ERRORLESS |Exercise JEE SECTION REASONING TYPE QUESTIONS|7 VideosCOORDINATION CHEMISTRY
ERRORLESS |Exercise JEE SECTION COMPREHENSION TYPE QUESTION (PASSAGE - I )|3 VideosCOORDINATION CHEMISTRY
ERRORLESS |Exercise JEE SECTION Only one choice answer|57 VideosCLASSIFICATION OF ELEMENTS AND PERIODIC PROPERTIES
ERRORLESS |Exercise JEE Section Matrix Match type questions|2 VideosD & P-BLOCK ELEMENTS
ERRORLESS |Exercise JEE Section (Numeric answer type questions)|2 Videos
ERRORLESS -COORDINATION CHEMISTRY-JEE SECTION MORE THAN ONE CHOICE CORRECT ANSWER
- Potassium manganate (K2MnO4) is formed when
Text Solution
|
- The aqueous solution of the following salts will be coloured in the ca...
Text Solution
|
- The compound(s) that exhibits(s) geometrical isomerism is/are
Text Solution
|
- For the given aqueous reactions, which of the statements(s) is ( are) ...
Text Solution
|
- The pair of coordination complex exhibiting the same kind of isomerism...
Text Solution
|
- Addition of excess aqueous ammonia to a pink coloured aqueous solution...
Text Solution
|
- Zn and Ag can be separated from each other by
Text Solution
|
- K(4)[Fe(CN)(6)] is used to detect
Text Solution
|
- Which of the following statements are correct about the following comp...
Text Solution
|
- Which of the following are square planar complexes
Text Solution
|
- Zinc is the only metal in group -12 which shows amphoteric properties ...
Text Solution
|
- A d-block element forms octahedral complex but its spin magnetic momen...
Text Solution
|
- Which of the following statement(s) is // are false
Text Solution
|