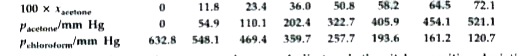Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTION
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS|15 VideosSOLUTION
U-LIKE SERIES|Exercise MULTIPLE CHOICE QUESTIONS|20 VideosSOLUTION
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION D)|2 VideosSOLID STATE
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|8 VideosSURFACE CHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST ( SECTION A)|7 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-SOLUTION-NCERT TEXTBOOK EXERCISES
- At 300 K, 36 g of glucose present in a litre of its solution has an os...
Text Solution
|
- Suggest the most important type of intermolecular attractive interacti...
Text Solution
|
- Based on solute-solvent interactions, arrange the following in order o...
Text Solution
|
- Amongst the following compounds, identify which are insoluble, partial...
Text Solution
|
- If the density of some lake water is 1.25 g mL^(-1) and contains 92 g...
Text Solution
|
- If the solubility product of CuS is 6 xx 10^(-16) , calculate the maxi...
Text Solution
|
- Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile (CH3...
Text Solution
|
- Nalorphene (C19H21NO3) , similar to morphine, is used to combat withdr...
Text Solution
|
- Calculate the amount of benzoic acid (C6H3COOH) required for preparin...
Text Solution
|
- The depression in freezing point of water observed for the same amount...
Text Solution
|
- Calculate the depression in the freezing point of water when 10 g of C...
Text Solution
|
- 9.5 g of CH2 FCOOH is dissolved in 500 g of water. The depression in ...
Text Solution
|
- Vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapou...
Text Solution
|
- Henry's law constant for the molality of methane in benzene at 298 K i...
Text Solution
|
- 100 g of liquid A (molar mass 140 g "mol"^(-1) ) was dissolved in 1000...
Text Solution
|
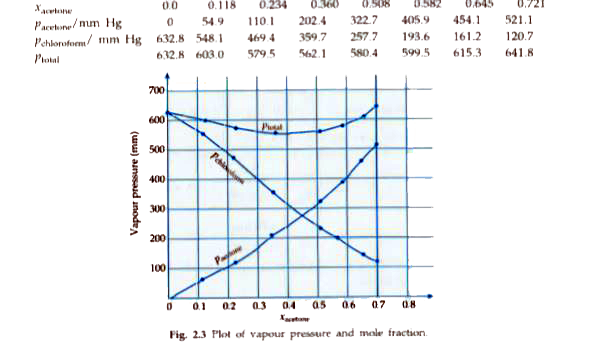
- Vapour pressures of pure acetone and chloroform at 328 K are 741.8 mm ...
Text Solution
|
- Benzene and toluene form ideal solution over the entire range of compo...
Text Solution
|
- The air is a mixture of a number of gases. The major components are ox...
Text Solution
|
- Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of wat...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 25...
Text Solution
|