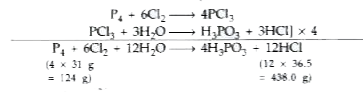Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise LONG ANSWER QUESTION-II|68 VideosTHE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST ( SECTION A)|7 VideosTHE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SHORT ANSWER QUESTIONS|87 VideosTHE D-AND F-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)|7 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-THE P-BLOCK ELEMENTS -LONG ANSWER QUESTION-I
- Account for the following: SbF5 is much more stable than BiF5 .
Text Solution
|
- Account for the following : Chlorine water has both oxidising and bl...
Text Solution
|
- Account for the following : H3PO2 and H3PO3 act as good reducing age...
Text Solution
|
- Account for the following : On addition of ozone gas to KI solution...
Text Solution
|
- Write down the equations for the hydrolysis of XeF4 and XeF6. Which of...
Text Solution
|
- Write chemical equations for the following processes : Chlorine rea...
Text Solution
|
- Write chemical equations for the following processes : Orthophospho...
Text Solution
|
- Write chemical equations for the following processes : PtF6 and Xe ...
Text Solution
|
- Complete the following chemical equations : Ca3P2(s) + H2O (l) to
Text Solution
|
- Complete the following chemical equations : Cu^(2+) (aq) + NH3 (aq)...
Text Solution
|
- Complete the following chemical equations : F2(g) + NH3(aq) to
Text Solution
|
- On reaction with Cl2, phosphorus forms two types of halides A and B. H...
Text Solution
|
- Explain why the stability of oxoacids of chlorine increases in the ord...
Text Solution
|
- P4O(6) reacts with water according to equation P4O6 + 6H2O to 4H3PO3 ....
Text Solution
|
- White phosphorus reacts with chlorine and the product hydrolysis in th...
Text Solution
|
- Name three oxoacids of nitrogen. Write the disproportionation reaction...
Text Solution
|
- Give an example to show the effect of concentration of nitric acid on ...
Text Solution
|
- Give reasons for the following: Addition of Cl2 to KI solution giv...
Text Solution
|
- Give reasons for the following: Phosphinic acid behaves as a mono...
Text Solution
|
- Give reasons for the following: White phosphorus is much more reac...
Text Solution
|