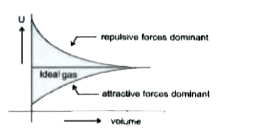
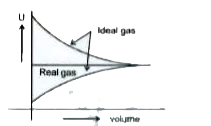
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Practice Sheet- Exercise-II|30 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Practice Sheet- Exercise-III|30 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Lecture Sheet -Exercise-IV|16 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 4 (Assertion (A) & Reason (R) Type Questions)|44 VideosCHEMISTRY IN EVERY DAY LIFE
AAKASH SERIES|Exercise PRACTICE EXERCISE|29 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-Practice Sheet-Exercise-I
- Which of the following diagram correctly represents the variation of i...
Text Solution
|
- One mole of an ideal monoatomic gas expands isothermally against const...
Text Solution
|
- The magnitudes of enthalpy changes for irreversible adiabatic expansio...
Text Solution
|
- A sample of gas is compressed from an initial volume of 2v(0) " to " v...
Text Solution
|
- One mole of an ideal monoatomic gas at temperature T and volume 1L exp...
Text Solution
|
- Which has maximum internal energy at 298K?
Text Solution
|
- A heat engine absorbs heat q(1) from a source at temperature T(1) and ...
Text Solution
|
- Assertion: An isothermal process is always an isolated one. Reason: ...
Text Solution
|
- What is DU for the process described by figure. Heat supplied during t...
Text Solution
|
- 6 what is the change in internal energy when a gas contracts from 377 ...
Text Solution
|
- The heat capacity of liquid water is 75.6 J/mol K, while the enthalpy ...
Text Solution
|
- Statement -I : There is no change in enthalpy of an ideal gas during c...
Text Solution
|
- Molar heat capacity of water in equilibrium with ice at constant press...
Text Solution
|
- Which of the following statements is false?
Text Solution
|
- On which of the following factors does internal energy depend upon
Text Solution
|
- The enthalpy is maximum for
Text Solution
|
- The different between DeltaH and DeltaE for the reaction BaCl(2(aq)) ...
Text Solution
|
- For the gaseous reaction involving the complete combustion of isobutan...
Text Solution
|
- During a process work equivalent to 400J is done on a system, which gi...
Text Solution
|
- A system absorbs 10kJ of heat at constant volume and its temperature r...
Text Solution
|