Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-I)|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-II )|3 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Very Short Answer Type Questions )|4 VideosII PUC TOPPER'S ANSWER MARCH - 2017
OSWAAL PUBLICATION|Exercise Part - D|11 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - II)|3 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN-CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Short Answer Type Questions )
- Which is more basic among aqueous solutions of aniline and ammonia ? G...
Text Solution
|
- How do you convert ethane nitrite to ethanamine ? Give equation.
Text Solution
|
- Explain the mechanism of nitration of benzene .
Text Solution
|
- What is zwitter ion ? Write zwitter ion structure of Alanine.
Text Solution
|
- What happens when aniline is heated with chloroform and alcoholic pota...
Text Solution
|
- Explain the following ructions : (a) Gabriel Phthalimide reaction ...
Text Solution
|
- Give chemical tests to distinguish between the following pairs of comp...
Text Solution
|
- Give reasons : (i) Aniline is a weaker base than cyclohexyl amine. ...
Text Solution
|
- Account for the following : (i) Diazonium salts of aromatic amines ...
Text Solution
|
- Distinguish between : (i) Methyl amine and dimethyl amine, (ii) An...
Text Solution
|
- Describe the following giving the relevant chemical equation in each c...
Text Solution
|
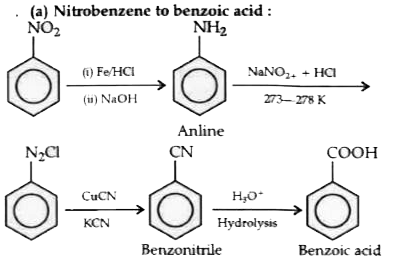
- Bring about the following conversions : Nitrobenzene to benzoic acid
Text Solution
|
- Bring about the following conversions : Benzyl chloride to 2-phenyl ...
Text Solution
|
- Give the structure of A, B and C In the following reactions : CH(3)B...
Text Solution
|
- Give the structure of A, B and C In the following reactions : CH(3)C...
Text Solution
|
- How will you convert the following : (i) Nitrobenzene into aniline ...
Text Solution
|
- Explain why an alkyl amine is more basic than ammonia.
Text Solution
|
- How would you convert : (i) Aniline to nitrobenzene , (ii) Aniline...
Text Solution
|