Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
OSWAAL PUBLICATION|Exercise Topic - 2 (ORDER OF A REACTION , INTEGRATED RATE EQUATIONS AND HALF LIFE OF A REACTION ) (Short Answer Type Questions)|8 VideosCHEMICAL KINETICS
OSWAAL PUBLICATION|Exercise Topic - 2 (ORDER OF A REACTION , INTEGRATED RATE EQUATIONS AND HALF LIFE OF A REACTION ) ( Long Answer Type Questions - I)|17 VideosCHEMICAL KINETICS
OSWAAL PUBLICATION|Exercise Topic - 1(RATE OF CHEMICAL REACTION AND FACTORS AFFECTING RATE OF REACTION ) (Long Answer Type Questions - I)|3 VideosBIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC -II (LONG ANSWER TYPE QUESTIONS)|2 VideosCHEMISTY IN EVERYDAY LIFE
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS-I|1 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CHEMICAL KINETICS-Topic - 2 (ORDER OF A REACTION , INTEGRATED RATE EQUATIONS AND HALF LIFE OF A REACTION ) (Very Short Answer Type Questions)
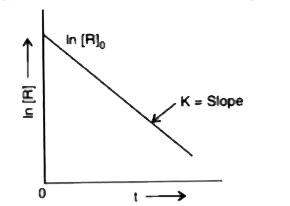
- From the following plot, predict the order of the reaction.
Text Solution
|
- What is the order for the reaction 2NH(3)(g) overset(1130K//Mo)rarr N(...
Text Solution
|
- For the reaction A+B rarr products. The rate becomes doubled when conc...
Text Solution
|
- If the rate constant of a reaction is k = 3 xx 10^(-4) s^(-1), then id...
Text Solution
|
- Write the unit of rate constant for a zero order reaction.
Text Solution
|
- Define "Order of a reaction".
Text Solution
|
- Rate constant for a reaction is 1.85 xx 10^(2)s^(-1). Give the order o...
Text Solution
|
- In Which order of reaction, rate of reaction becomes equal to specific...
Text Solution
|
- For a reaction , A + B rarr product, the rate law is given by r = k[A]...
Text Solution
|