Text Solution
Verified by Experts
Topper's Solved these Questions
D - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC-1 (D - BLOCK ELEMENTS, THEIR PROPERTIES AND COMPONDS) LONG ANSWER TYPE QUESTIONS - I|20 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC-1 (D - BLOCK ELEMENTS, THEIR PROPERTIES AND COMPONDS)LONG ANSWER TYPE QUESTIONS - II|12 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)|6 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Answer Type Questions)|19 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (LONG ANSWER TYPE QUESTIONS)|4 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-D - BLOCK ELEMENTS F - BLOCK ELEMENTS -TOPIC-1 (D - BLOCK ELEMENTS, THEIR PROPERTIES AND COMPONDS)(SHORT ANSWER TYPE QUESTIONS - I)
- Explain briefly how +2 state becomes more and more stable in the first...
Text Solution
|
- Name be oxo-metal anions of the first series of the transition metals ...
Text Solution
|
- What are the characteristics of the transition elements and why are th...
Text Solution
|
- In what way is the electronic configuration of transition elements dif...
Text Solution
|
- How is potassium permanganate ( KMnO4) prepared from MnO2 ? write the ...
Text Solution
|
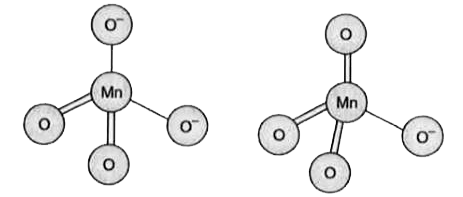
- Write the structure of MnO4^(2-) and MnO(4)^(-) ions.
Text Solution
|
- Explain why: (i) E^0 for Mn^(3+)//Mn^(2+) couple is more positive th...
Text Solution
|
- Complete the following chemical equations: (i) MnO(4)^(-) (aq) + S2O...
Text Solution
|
- Assign reasons for the following: (i) Copper (I) ion is not known in...
Text Solution
|
- Assign reasons for each of the following: (i) Transition metals gene...
Text Solution
|
- How would you account for the following: (i) Cr^(2+) is reducing in ...
Text Solution
|
- State reasons for the following: (i) Cu(I) ion is not stable in an a...
Text Solution
|
- Explain giving reasons : (i) Transition metals and their compounds g...
Text Solution
|
- Account for the following: (i) Cu^(+) ions are not stable in aqueous s...
Text Solution
|
- How would you account for the following: (i) The atomic radii of the...
Text Solution
|
- Name the reaction that takes place when a mixture of potassium dichrom...
Text Solution
|
- Account for the following: (i) In the series Sc to Zn, the enthalpy ...
Text Solution
|
- Account for the following: (i) Transition elements exhibit higher en...
Text Solution
|
- Explain the following observations: (i) Generally, there is an incr...
Text Solution
|
- Explain the following observations: (i) Transition elements generall...
Text Solution
|