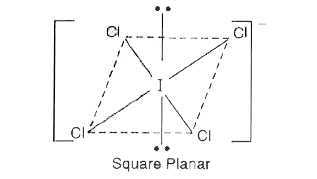
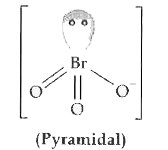
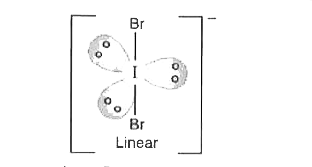Text Solution
Verified by Experts
Topper's Solved these Questions
P - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(VERY SHORT ANSWER TYPE QUESTIONS)|36 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(SHORT ANSWER TYPE QUESTIONS)|7 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 3 (GROUP - 17 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(SHORT ANSWER TYPE QUESTIONS)|11 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-II )|3 VideosPOLYMERS
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS - II|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-P - BLOCK ELEMENTS-TOPIC - 3 (GROUP - 17 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)
- (a) Give two reasons for the anomalous behaviour of fluorine. (b) Give...
Text Solution
|
- (i) How is chlorine prepared by using MnO(2) (ii) Complete the react...
Text Solution
|
- Complete the following equations. (i) 2F(2)+2H(2)Orarr (ii) H(2)+C...
Text Solution
|
- Name the gas liberated when concentrated HCI is heated with MnO(2) Giv...
Text Solution
|
- Mention any two resons for anomalous behaviour of Fluorine.
Text Solution
|
- Inter halogen compounds are more reactive than halogens . Why ?
Text Solution
|
- Which is the strongest acid among the hydrogen halides? Give one reaso...
Text Solution
|
- Complete the following equation : i) underset("(Cold and dilute)")(2...
Text Solution
|
- With the help of a neat diagram, describe the manufacture of caustic s...
Text Solution
|
- Give the formula and describe the structure of a noble gas species whi...
Text Solution
|
- How are the following interhalogen compounds prepared? (i) ClF(3) (ii...
Text Solution
|
- Complete the following equatious: i) 2Al+3Cl(2)rarr ii) H(2)S+Cl(2...
Text Solution
|
- Account for the following : (i) IC l is more reactive than I(2). (...
Text Solution
|
- Name the types of interhalogen compounds ? Give examples.
Text Solution
|