Text Solution
Verified by Experts
Topper's Solved these Questions
P - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(SHORT ANSWER TYPE QUESTIONS)|7 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)|3 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 3 (GROUP - 17 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)|14 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-II )|3 VideosPOLYMERS
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS - II|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-P - BLOCK ELEMENTS-TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(VERY SHORT ANSWER TYPE QUESTIONS)
- What prompted Bartlett to the discovery of noble gas compounds.
Text Solution
|
- Noble gases have very high ionisation enthalpy. Why ?
Text Solution
|
- Ionisation enthalpy decreases down the group in noble gases. Why ?
Text Solution
|
- How does atomic radii changes in group - 18 elements ?
Text Solution
|
- Noble gases have large positive values of electron gain enthalpy. Why ...
Text Solution
|
- Name the first noble gas compound prepared by Neil Bartlett?
Text Solution
|
- Give common names of noble gases.
Text Solution
|
- How is XePtF(6) prepared ?
Text Solution
|
- How is radon obtained ?
Text Solution
|
- What is the percentage of noble gases in the atmospheric air ?
Text Solution
|
- Which noble gas in most abundant in atmospheric dry air?
Text Solution
|
- Why noble gases are called rare gases ?
Text Solution
|
- Why are the elements of Group - 18 known as noble gases?
Text Solution
|
- Why the noble gases are called inert gases ?
Text Solution
|
- Write the general electronic configuration of noble gases.
Text Solution
|
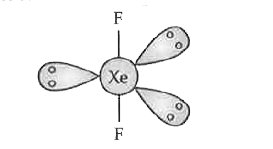
- Draw the structure of XeF(2) molecule.
Text Solution
|
- What inspired N. Bartlett for carrying out reaction between Xe and PtF...
Text Solution
|
- Helium is used in diving equipment.
Text Solution
|
- Draw the molecular structure of XeF(6).
Text Solution
|
- Complete the following chemical equation - XeF(4)+SbF(5)rarr
Text Solution
|