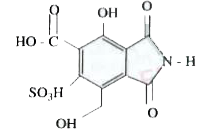
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-4 (Single Answer questions)|6 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-4 (More than one correct answer questions)|7 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-3 (Matching Type Questions)|2 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Practice Sheet-5|30 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOXYLIC ACIDS -PRACTICE SHEET-3 (Integer Type questions)
- How many of the following are stronger than HCOOH (formic acid) ? Ph...
Text Solution
|
- How many of the following dicarboxylic acids are forming cyclic anhydr...
Text Solution
|
- <br? Final product (X) Degree of unsaturation of final product is
Text Solution
|
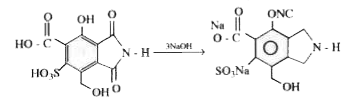
- How many moles of NaOH would be required for complete Neutralisation o...
Text Solution
|
- How many moles of CO2 will released when following compound treated wi...
Text Solution
|